Anti-SERINC5 antibody and application thereof
An antibody and monoclonal antibody technology, applied in the field of molecular biology, can solve problems such as lack of and hinder research progress, and achieve the effect of accelerating research progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Screening of the polypeptide immunogen of SERINC5
[0049] SERINC5 (GENBANK ACCESSION: NM_001174072) is a nine-transmembrane protein, and it is not yet possible to prepare a purified protein. Therefore, the strategy of preparing monoclonal antibodies is to immunize mice with peptides.
[0050] The extracellular region of SERINC5 includes the amino terminal and 4 extracellular loops, of which the amino terminal contains 35 amino acids; the first extracellular loop contains 14 amino acid residues and contains 1 glycosylation site; the second extracellular loop The loop contains 21 amino acid residues and contains 1 glycosylation site; the third extracellular loop contains only 7 amino acids; the fourth extracellular loop consists of 53 amino acid residues and has no glycosylation site . The amino terminal and the fourth extracellular loop region of SERINC5 contain more amino acid residues and no glycosylation sites, making it easier to screen out suitable polyp...
Embodiment 2
[0054] Embodiment 2 Preparation of anti-human SERINC5 monoclonal antibody
[0055] 1. Immunization of animals
[0056] The KLH-coupled polypeptide (SEQ ID NO:9) was dissolved in distilled water to prepare a concentration of 1 mg / ml as an immunogen, and 100 μl of the immunogen and complete Freund's adjuvant (Sigma-Aldrich) were mixed and shaken to form a water-in-oil emulsion . For the first immunization, 6-8 week-old BALB / c mice were subcutaneously injected with 200 μl of immunogen / adjuvant mixture. After the initial immunization, a half-dose booster immunization was carried out at the 3rd week and 6th week respectively, and blood was collected at the 8th week, and the antibody titer was analyzed by ELISA.
[0057] 2. Cell Fusion
[0058] Two weeks after the last booster immunization, the mice were sacrificed, spleen cells were taken, mixed with SP2 / 0 cells (purchased from ATCC, product number: CRL-1581) at a ratio of 10:1, centrifuged and washed with PEG1450 (Sigma -Aldri...
Embodiment 3
[0061] Example 3 Detection of cell surface SERINC5 by flow cytometry
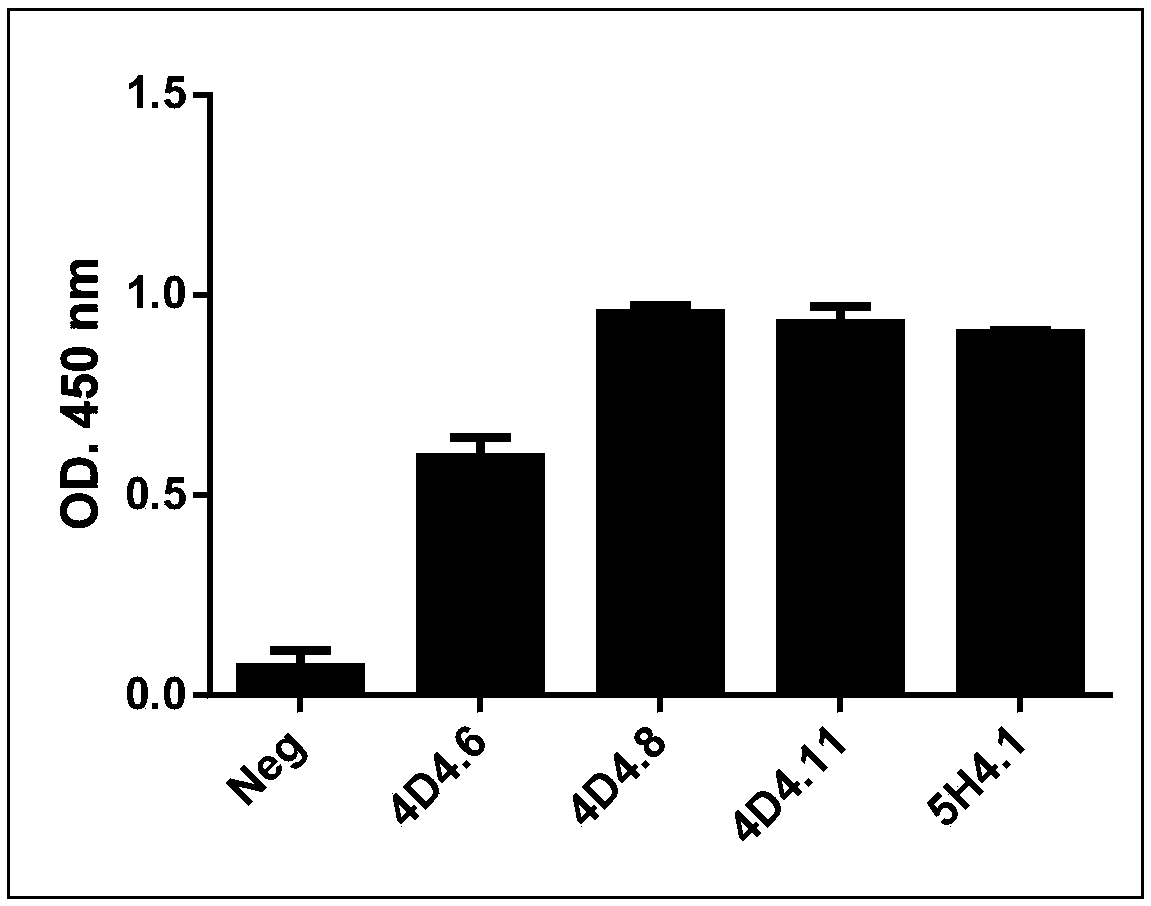
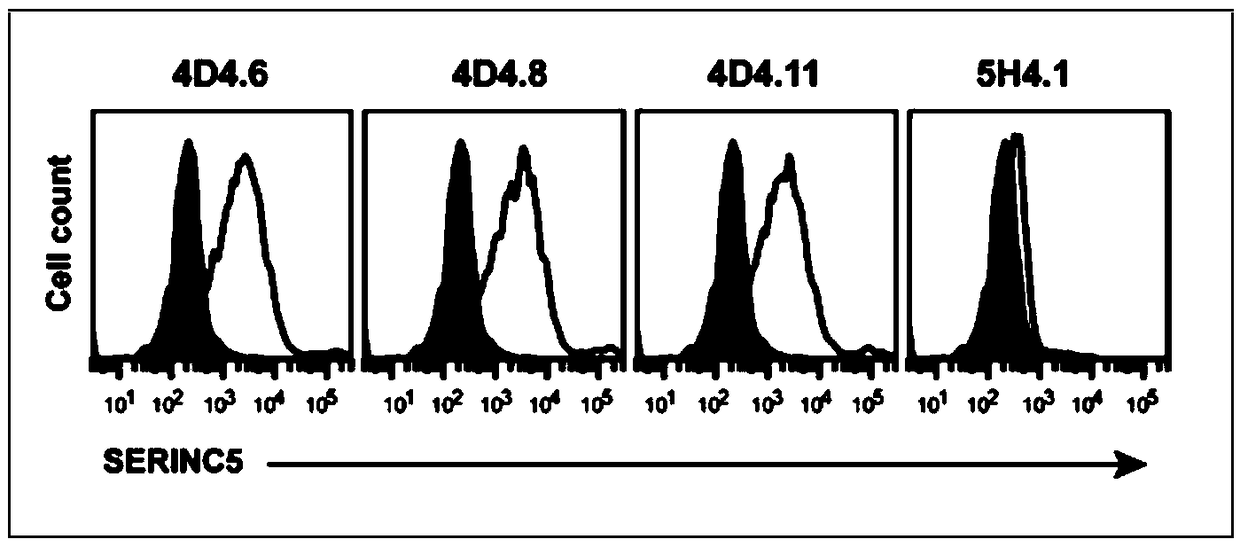
[0062] Take 100 μl of the culture supernatant of the hybridoma cells 4D4.6, 4D4.8, 4D4.11, and 5H4.1 screened in Example 2, and mix with 1×10 6 A 293T cell and 293T-SERINC5 cell (293T cell line stably expressing human SERINC5) (the above two cell lines were preserved by the Institute of Liver Diseases, Shenzhen Third People's Hospital) were incubated at room temperature for 10 minutes, washed with PBS and centrifuged. 0.2 μl of fluorescent secondary antibody AF647-Anti mouse IgG (H+L) (Invitrogen, Cat. No. A-21237), incubated at room temperature in the dark for 10 minutes, washed with PBS, and flow cytometry to detect the fluorescent signal on the cell surface. It was found that 4D4.6, 4D4.8, and 4D4.11 can recognize SERINC5 molecules on the cell surface, while 5H4.1 can only recognize polypeptides, but not SERINC5 molecules on the cell surface ( Figure 4 , the gray peak in the figure is the result of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com