Transaminase, mutant and application thereof in production of L-glufosinate ammonium
The technology of transaminase and mutant is applied in the application field of preparing asymmetric chiral amine compounds, and can solve the problems of low stereoselectivity, high catalyst price, difficult recovery of solvent and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
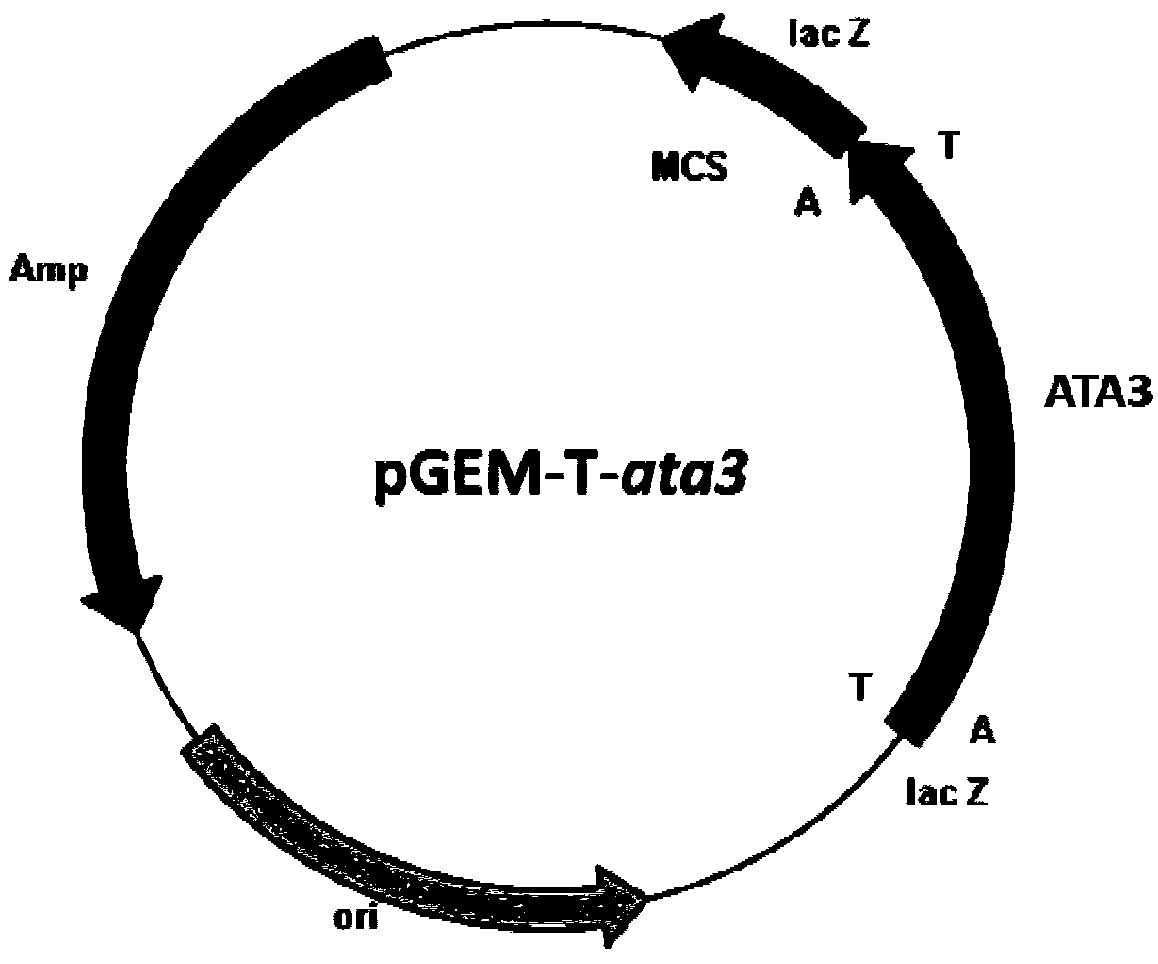
[0032] Embodiment 1: the amplification of transaminase gene ata3
[0033] Pseudomonas fluorescens ZJB09-108 is isolated from the soil and stored in the China Center for Type Culture Collection (preservation number CCTCC NO: M2012539, which has been disclosed in the patent application, application number CN 201210593105.3, published (announcement ) No. CN103131649A).
[0034] According to the transaminase gene sequencing information from Pseudomonas (WP_076423369.1) included in Genbank, the total genomic DNA of Pseudomonas CCTCC NO: M2012539 was extracted with a rapid nucleic acid extraction instrument, and the genomic DNA was used as a template. 1 (5'-ATGTCTAAAAACGAATCTCTGCTGCAGC-3') and primer 2 (5'-TTAAGCCAGTTCGTCGAAGCATTC-3') for PCR amplification. PCR reaction system (total volume 50 μL): 5 μL of 10×Pfu DNA Polymerase Buffer, 1 μL of 10 mM dNTP mixture (2.5 mM each of dATP, dCTP, dGTP, and dTTP), 1 μL of cloning primer 1 and primer 2 each at a concentration of 50 μM, geno...
Embodiment 2
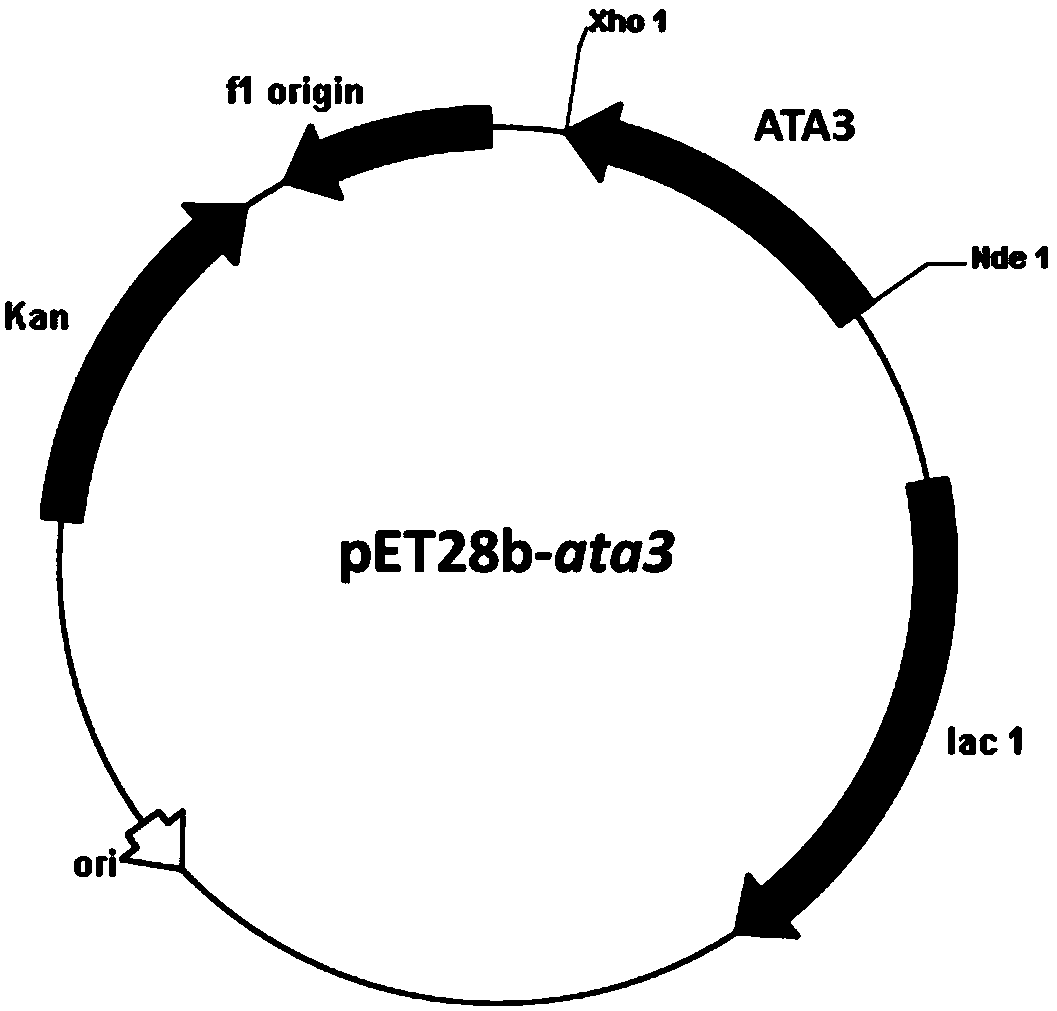
[0037] Embodiment 2: Construction of recombinant Escherichia coli E.coli BL21(DE3) / pET28b-ata3
[0038] Design primer 3 (5'-CCG according to embodiment 1ata3 gene sequence CATATG TCTAAAAACGAATCTC-3'), primer 4 (5'-TTG CTCGAG TTAAGCCAGTTCGTCG-3'), and Nde I and Xho I restriction enzyme sites (underlined) were introduced into primer 3 and primer 4, respectively. Under the priming of primers 3 and 4, high-fidelity Pfu DNA polymerase was used to amplify, and the recombinant plasmid pMD18-T-ata3 was used as a template (obtained in Example 1) to obtain the ATA3 gene sequence, which was sequenced using NdeI and Xho The amplified fragment was treated with I restriction endonuclease (TaKaRa), and the fragment was ligated with the commercial vector pET28b (Invitrogen) treated with the same restriction endonuclease using T4 DNA ligase (TaKaRa) to construct an expression Vector pET28b-ata3 ( figure 2 ). The constructed expression vector pET28b-ata3 was transformed into Escherichia c...
Embodiment 3
[0039] Example 3: Induced expression of transaminase (ata3)
[0040] The recombinant Escherichia coli E. coli BL21(DE3) / pET28b-ata3 obtained in Example 2 was inoculated into LB liquid medium containing 50 μg / ml kanamycin resistance, cultivated at 37° C. for 12 hours at 200 rpm, and then added with 1% (v / v) Inoculum amount was inoculated into fresh LB liquid medium containing 50 μg / ml kanamycin resistance, and cultivated at 37°C and 150 rpm until the cell OD 600 After reaching 0.6-0.8, add IPTG with a final concentration of 0.1mM, induce culture at 25°C for 18h, centrifuge at 8000rpm at 4°C for 20min, discard the supernatant, collect the precipitate, and obtain the recombinant Escherichia coli E. coli containing the expression recombinant plasmid. coli BL21 / pET28b-ata3 wet cells. The bacteria can be directly used as a biocatalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com