Two-dimensional dinuclear zinc coordination polymer and preparation method and application thereof
A technology of zinc coordination polymer and polytetrafluoroethylene tube, which is applied in the field of coordination polymers, can solve problems such as use restrictions, and achieve the effect of simple preparation process and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation of Zinc Coordination Polymer
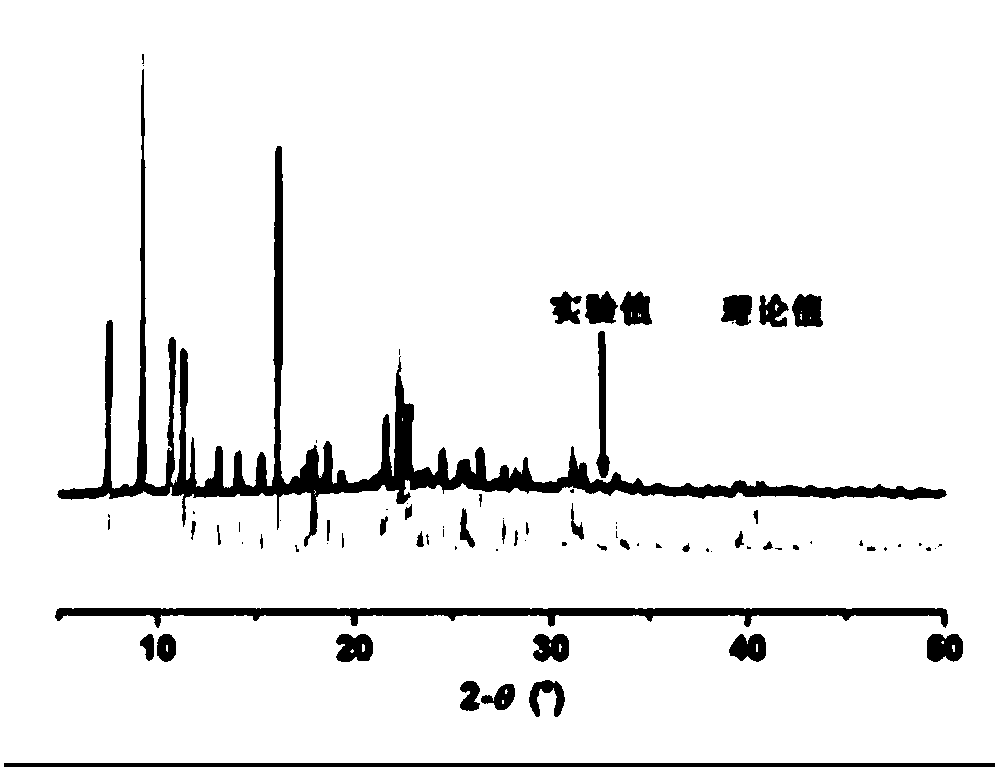
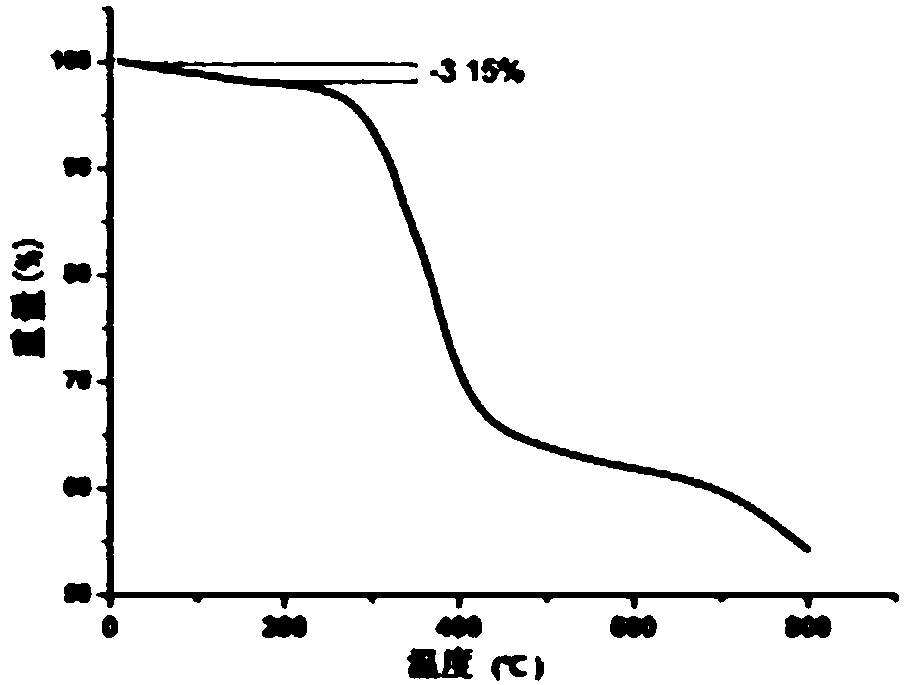
[0021] Weigh 0.2mmol H 3 L1, 0.2mmol L2 and 0.1mmol Zn(NO 3 ) 2 ·6H 2 O was added containing 6mL H 2 In a 13 mL polytetrafluoroethylene tube with O, slowly add KOH with a concentration of 0.2 mol / l dropwise into the stirring mixture to adjust the pH to 5, and continue stirring for 30 minutes. Seal the polytetrafluoroethylene tube in a stainless steel reaction kettle, heat it at 413K for three days, then cool it down to room temperature by 10°C every hour, and a colorless blocky crystal can be precipitated, washed with water and dried in vacuum, with a yield of 80%. Elemental analysis: theoretical value: C 57.47, H 3.19, N 4.97%; experimental value: C 57.93, H 3.39, N 5.14%.
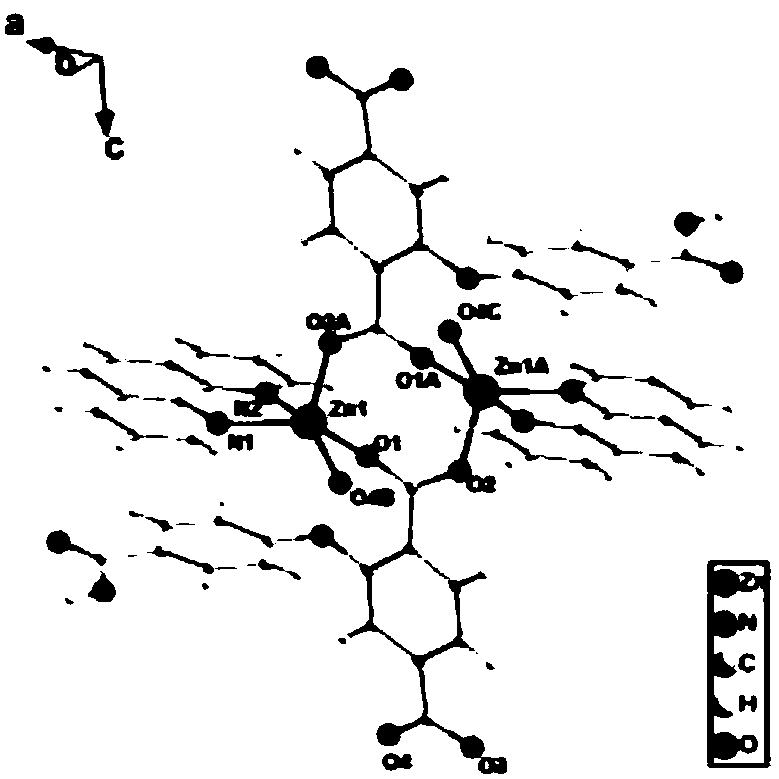
[0022] Zinc coordination polymer crystal structure determination:
[0023] The crystal structure was determined by X-ray diffraction, using Bruker D8Venture detector to monochromatize Mo-Kα rays through graphite monochromator, scanning mode ω, and th...
Embodiment 2
[0031] Example 2 The zinc coordination polymer of the present invention selectively recognizes dichromate ions and chromate ions in water.
[0032] First, prepare 10mL concentration of 5×10 -3 mol / L of different anion solutions, (anion=F - , Cl - ,Br - ,I - , SO 4 2- ,SCN - ,CH 3 COO - ,ClO 4 - ,CO 3 2- ,HPO 4 2-,MoO 4 2- ,CrO 4 2- and Cr 2 o 7 2- ). 2 mg of the zinc coordination polymers were added to 10 mL of different anion solutions, treated with ultrasound for 30 minutes, and sedimented for three days to form a suspension of zinc coordination polymers incorporated with inorganic anions. Get the supernatant, under the condition that the excitation wavelength is 290nm, and the slit width is 10nm, measure its fluorescence emission spectrum intensity, as Figure 4 As shown, the coordination polymer has unique fluorescence selectivity for dichromate ion and chromate ion.
Embodiment 3
[0033] Example 3 The sensitivity of the zinc coordination polymer of the present invention to recognize dichromate ions and chromate ions in water.
[0034] Add 2 mg of the coordination polymer to 10 mL of water, ultrasonicate for 30 minutes, and settle for three days to form a uniform suspension of the zinc coordination polymer. Take 2mL of the supernatant, and gradually add dropwise to a concentration of 1×10 -3 mol / L of dichromate ion or acid ion, from Figure 4 It can be seen that under the excitation wavelength of 290nm, the suspension of the zinc coordination polymer shows very strong fluorescence at 370nm. Such as Figure 5 The titration curve shows that with the dichromate ion in the system ( Figure 5 a) or chromate ion ( Figure 5 b) As the concentration increases, the fluorescence of the solution decreases significantly. In addition, if Figure 6 As shown, at low concentrations, the quenching effect can be dealt with by the Stern-Volmer equation: I 0 / I=1+K ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com