Preparation method and applications of double-stage brain-targeting polymer micelle drug delivery system
A technology of polymer glue and drug delivery system, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. It is convenient for subsequent development and industrialization, the preparation method is simple, and the operation is easy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] A preparation method of a two-stage brain-targeted polymer micelle drug delivery system, the steps of which are:
[0055] A. Preparation of hydrophilic host molecules:
[0056] (1) Weigh 3 g of mono-(6-p-toluenesulfonyl)-cyclodextrin (6-OTs-β-CD), dissolve it in 20 mL of anhydrous ethylenediamine, and pour the mixed solution into a round bottom flask Placed in an oil bath to react. The reaction temperature is at 40 or 50 or 60 or 70 or 80 or 90 o C, the reaction time is 1 or 5 or 8 or 15 or 22 or 30 or 36 or 42 or 48 h. After the reaction, the reaction solution was dropped into 500 mL of acetone to obtain a precipitate, which was collected by suction filtration and dried in vacuo. The precipitate was purified by dissolution / reprecipitation twice, and dried in vacuo to obtain mono-6-ethylenediamine-β-cyclodextrin (M6-β-CD).
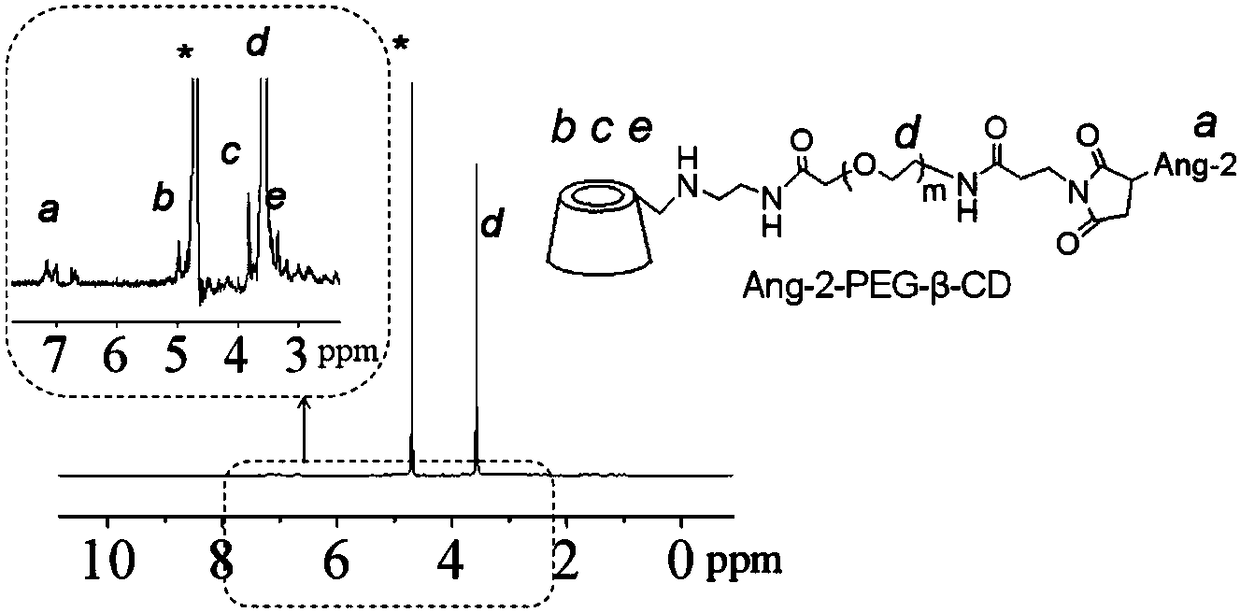
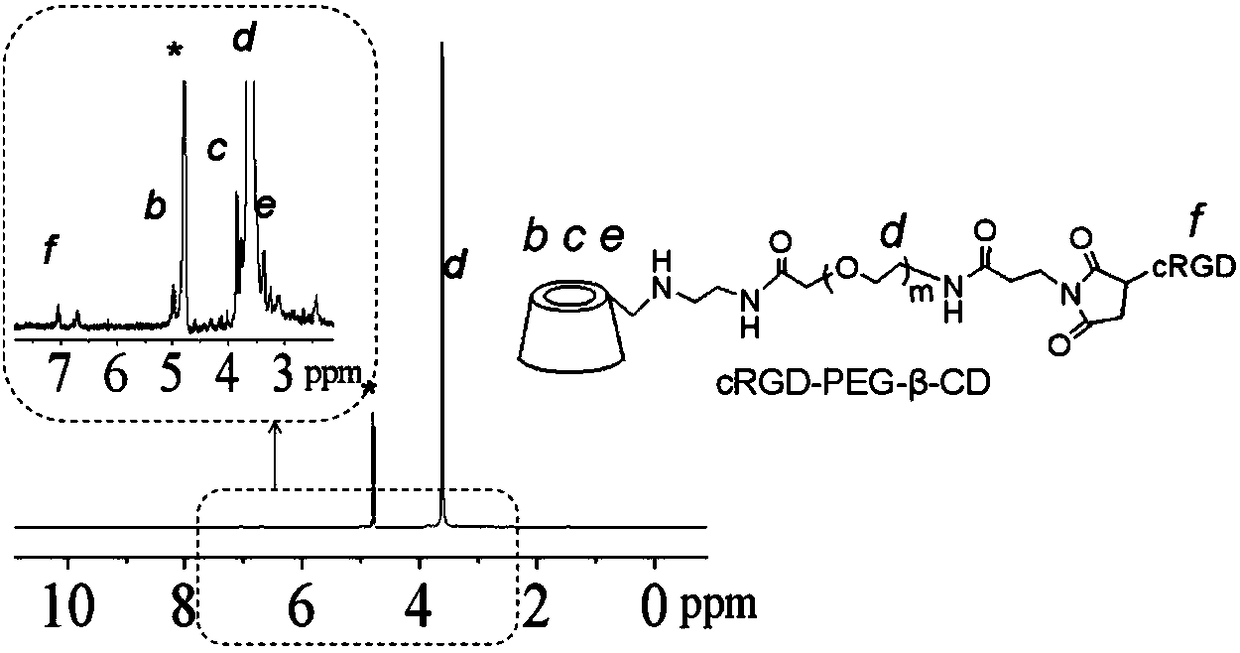
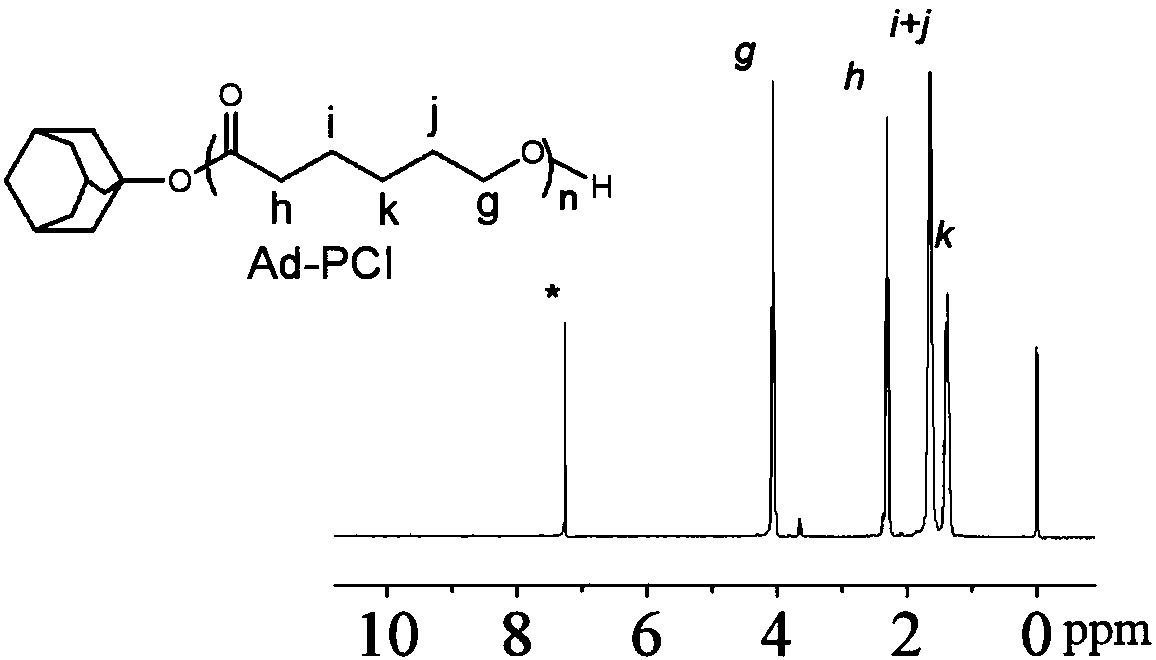
[0057] (2) Weigh 68.8 mg of mono-6-ethylenediamine-β-cyclodextrin (M6-β-CD) and maleimide-polyethylene glycol-succinimide ester (MAL-PEG-NHS )...
Embodiment 2
[0076] Application of a two-stage brain-targeting polymer micelle drug delivery system in the preparation of targeted drug carriers for the treatment or prevention of glioma (studies on anti-tumor activity in vivo and in vitro), the steps are:
[0077] A. Antitumor activity research in vitro:
[0078] (1) Cell uptake experiment
[0079] C6 cells were treated at 5.0×10 4 Cells / well were seeded into 24-well plates, and the cells were incubated in 1 mL DMEM containing 10% FBS for 24 hours. Then add free DOX solution, N-T micellar solution, D-T micellar solution (DOX content of 5 μg / mL) in fresh medium and incubate for 4 hours. After the cells were washed 3 times with PBS, fluorescent pictures of C6 cells were taken under a fluorescence microscope. At the same time, the cells were digested with trypsin and resuspended in PBS, and the uptake efficiency of the three materials by C6 cells was determined by flow cytometry.
[0080] The uptake efficiency of the three materials by b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com