Canine parvovirus vaccine composition, preparation method and application thereof
A technology of canine parvovirus and vaccine composition, applied in botany equipment and methods, biochemical equipment and methods, applications, etc., can solve the problems of attenuated virus strain mutation, susceptible to maternal antibody, immune failure, etc., and achieve good Immunogenicity, reduction and prevention of associated disease effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Construction of Canine Parvovirus VP2 Protein Kluyveromyces marx Recombinant Expression Vector
[0061] The amino acid sequence encoding canine parvovirus capsid protein VP2 is shown in SEQ ID No.1. According to the codon preference of Kluyveromyces marxis, the codon optimization of the VP2 gene sequence was carried out without changing the amino acid sequence. The optimized sequence is shown in SEQ ID No.2. Insert the optimized VP2 gene sequence into Kluyveromyces marx expression vector pUKD-N125 to construct the recombinant expression vector-PUKD-N125 / VP2, the specific process is as follows:
[0062] with specific primers
[0063] VP2-N125-F (SEQ ID No. 3)
[0064] 5' TTTTTTTGTT AGATCCGCGGATGAGTGACGGCGCAGTTC 3'
[0065] VP2-N125-R (SEQ ID No. 4)
[0066] 5'AGCTTGCGGCCTTAACTAGTTTAGTACAACTTTTCTAGGTG 3'
[0067] The VP2 gene was amplified by PCR, and the amplified product was subjected to 1% agarose gel electrophoresis to recover a 1755kb fragment, such as...
Embodiment 2
[0068] Example 2 Construction of Kluyveromyces marx genetically engineered bacteria Fim-1-pUKD-N125 / VP2
[0069] The yeast expression host strain used in the present invention is Kluyveromyces marxense Fim-1ura3Δ, and the preparation method of this strain can be constructed by the method disclosed in Example 1 of the Chinese patent publication CN105112313A, the auxotrophic strain Fim-1ura3Δ. Porcine parvovirus VP2 protein Kluyveromyces marx genetic engineering strain Fim-1-pUKD-N125 / VP2 is obtained by transforming the recombinant expression vector pUKD-N125 / VP2 to construct the Fim-1ura3Δ strain, the implementation process is as follows: The yeast Fim-1ura3Δ was inoculated in a glass test tube containing 3 mL of YEPD medium, and cultured overnight on a shaker at 30°C until the OD600 was 12-15. The cells were collected and washed with LiAc-TE solution (100 mM LiAc, 10 mM Tris-HCl pH 7.5, 1 mM EDTA). Add carrier DNA, recombinant expression vector pUKD-N125 / VP2, PEG solution (40...
Embodiment 3
[0071] Example 3 Expression of Canine Parvovirus VP2 Protein in Kluyveromyces marx
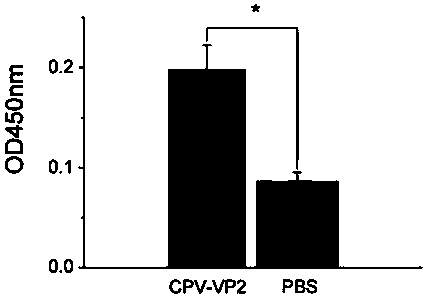
[0072] Recombinant engineered bacteria Fim-1-pUKD-N125 / VP2 and control bacteria Fim-1ura3Δ were respectively inoculated in 50ml of YD medium (1% yeast extract, 2% glucose), cultured at 30°C, 220rpm for 66h and then centrifuged The cells were collected, resuspended with 10 mM Tris-HCl (pH 7.5), and then homogeneously disrupted by high pressure. Take 100 μl of cell lysate and add protein electrophoresis loading buffer to mix, boil water for 5 min, then perform protein electrophoresis SDS-PAGE (polyacrylamide gel electrophoresis, PAGE) and Western Blot with CPV-VP2 antibody to detect the expression level of VP2 protein. The CPV-VP2 antibody used for Western Blot detection is a polyantibody serum isolated from mice immunized with PPV-VP2 protein recombinantly expressed in Escherichia coli, and the secondary antibody is a goat anti-mouse polyclonal antibody labeled with horseradish peroxidase.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dry cell weight | aaaaa | aaaaa |
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com