Novel composition of nucleoside amino phospholipid compound and preparation method thereof
A compound, hydrate technology, applied in the field of pharmaceutical preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
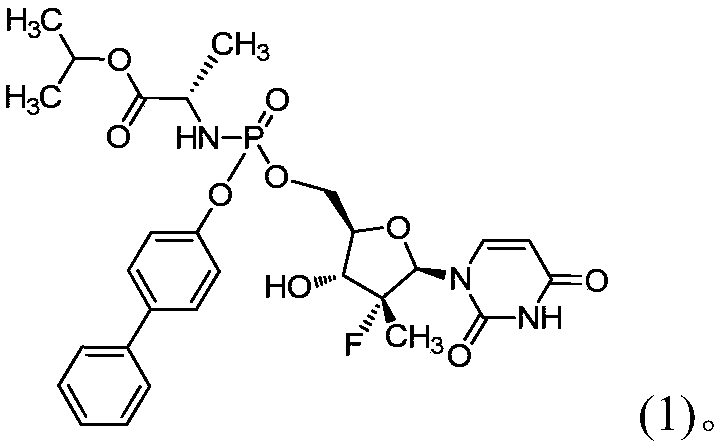
[0085] Example 1: (S)-2-[[[(S)-(1,1'-biphenyl-4-oxyl)]-[((2R,3R,4R,5R)-5-(2 ,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-3-hydroxyl-4-methyltetrahydrofuran-2-yl)methoxy]phosphoryl]amino] Preparation of isopropyl propionate
[0086]
[0087] Synthesis of Step 1 (S)-2-[[(1,1'-biphenyl-4-oxyl)(pentafluorophenoxy)phosphoryl]amino]propionic acid isopropyl ester
[0088]
[0089] In a 50L glass reactor, under the protection of nitrogen, add phosphorus oxychloride (1.53kg, 10mol) and dichloromethane (10L), stir, and cool down to below -30°C. A dichloromethane (5 L) solution of triethylamine (1.01 kg, 10 mol) was added dropwise, and the internal temperature was kept below -30°C during the dropwise addition. After the dropwise addition was completed, a solution of 4-hydroxybiphenyl (1.7kg, 10mol) in tetrahydrofuran (3.4L) was slowly added dropwise, and stirred for 30min after the dropwise completion. Control the internal temperature below -30°C, add L-alanine isopropyl hydro...
Embodiment 2
[0098] Example 2: (S)-2-[[[(S)-(1,1'-biphenyl-4-oxyl)]-[((2R,3R,4R,5R)-5-(2 ,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-3-hydroxyl-4-methyltetrahydrofuran-2-yl)methoxy]phosphoryl]amino] Confirmation of the configuration of isopropyl propionate
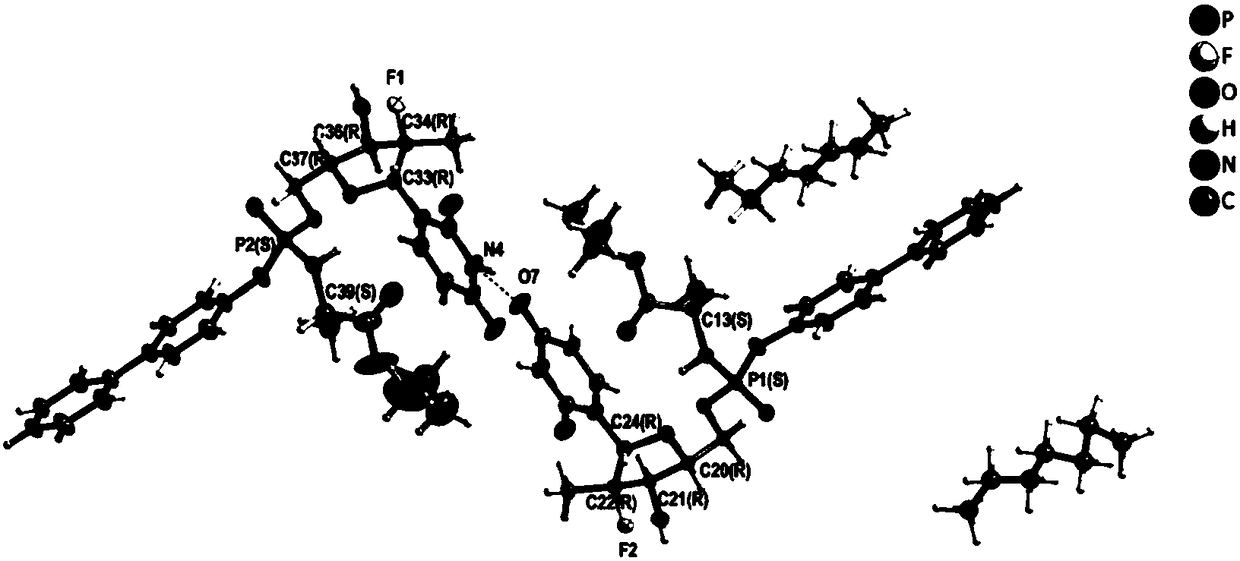
[0099] Weigh 15 mg of the compound of formula 1a prepared in Example 1 into a 3 mL vial, add 5 mL of dichloromethane / n-heptane mixed solvent system with a volume ratio of 2:1, shake the obtained clear solution, and cover the vial with a parafilm Prick holes on it and place it at room temperature for 6 days to obtain crystals.
[0100] The single crystal sample obtained by the above method is collected by X-ray single crystal diffraction data and its single crystal structure is analyzed. Table 1 lists the single crystal structure data and structure refinement parameters of the n-heptane solvate of the compound of formula 1a. Single crystal structure analysis has determined the absolute configuration of the chiral center in the c...
Embodiment 3
[0103] Example 3 (S)-2-[[[(S)-(1,1'-biphenyl-4-oxyl)]-[((2R,3R,4R,5R)-5 in solid oral form -(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-3-hydroxyl-4-methyltetrahydrofuran-2-yl)methoxy]phosphoryl ]Amino]propionate Isopropyl Preparation
[0104] Table 2. Prescription
[0105] Compound of formula (1a)
25.64
Crospovidone
38.46
15.38
12.82
7.69
total
100.00
[0106] Unit: % by weight (w / w).
[0107] Preparation steps:
[0108] a. mix the compound of formula (1a) with prescription amount, crospovidone and microcrystalline cellulose in a wet granulator, and add an appropriate amount of ethanol to prepare wet granules;
[0109] b. Dry the wet granules;
[0110] c. mixing the dried granules with lactose and croscarmellose sodium;
[0111] d. Fill the mixed granules into capsules.
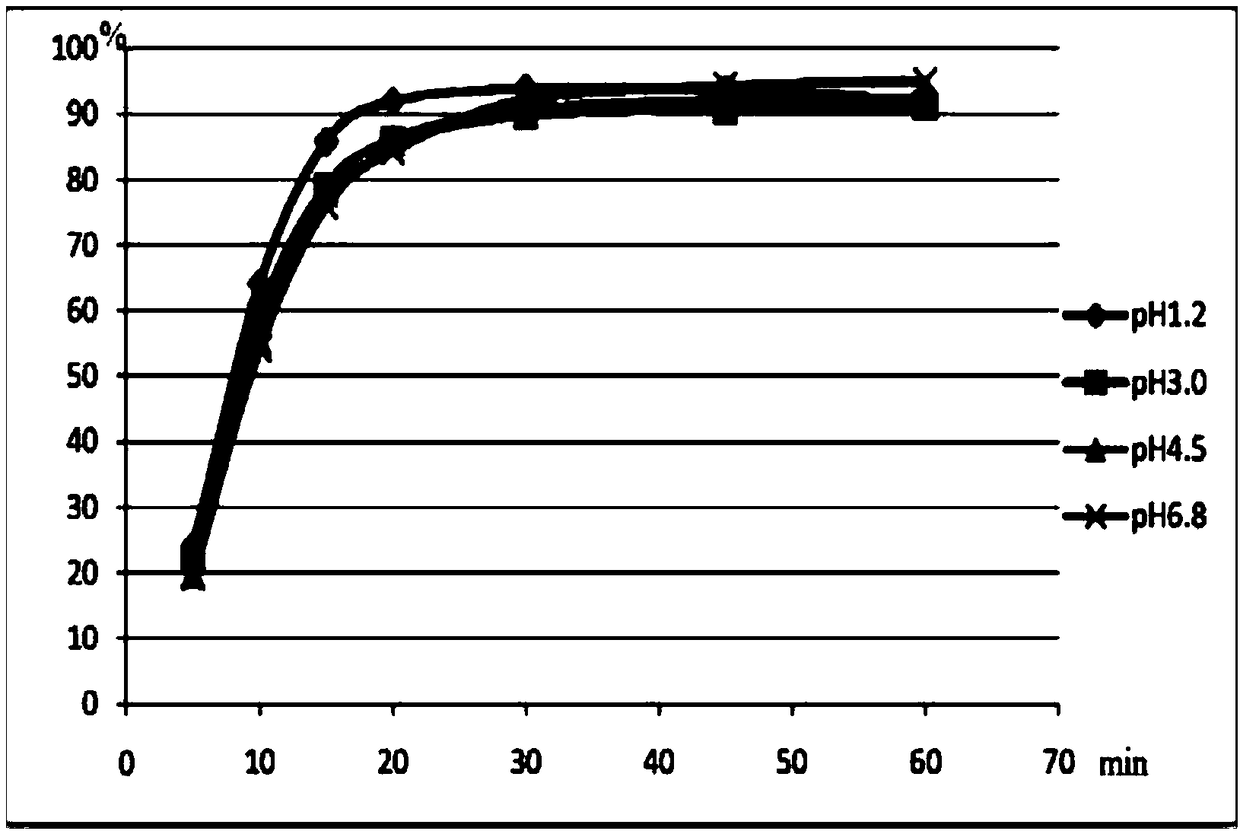
[0112] The formulation of this embodiment can disintegrate rapidly an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com