Light-cured organic silicon resin and preparation method thereof, photosensitive resin and application
A technology of photosensitive resin and silicone, applied in the direction of additive processing, etc., can solve the problems of long reaction time, slow curing rate, high energy consumption, etc., to improve curing rate, reduce molding volume shrinkage and warpage, and improve curing performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The present invention also provides a method for preparing the above photocurable silicone resin, comprising the following steps:
[0033] At least one of alkyl-containing siloxane and aromatic-containing siloxane is mixed with epoxy-containing siloxane and unsaturated double bond-containing siloxane with a catalyst and a solvent at a temperature of 60 At ~80°C, react with mechanical stirring for 6-8 hours to obtain a light-curable silicone resin.
[0034] In the invention, firstly, the catalyst and the solvent are mixed and stirred to obtain a mixed solution. Wherein, the solvent is selected from ethanol and deionized water. The mixing speed is 200-300rpm, and the mixing temperature is 20-30°C.
[0035] Next, at least one of the alkyl-containing siloxane and the aromatic-containing siloxane, the epoxy-containing siloxane and the siloxane containing unsaturated double bonds are mixed with the above-mentioned mixed solution. Under 60-80°C, mechanically stir and react ...
Embodiment 1
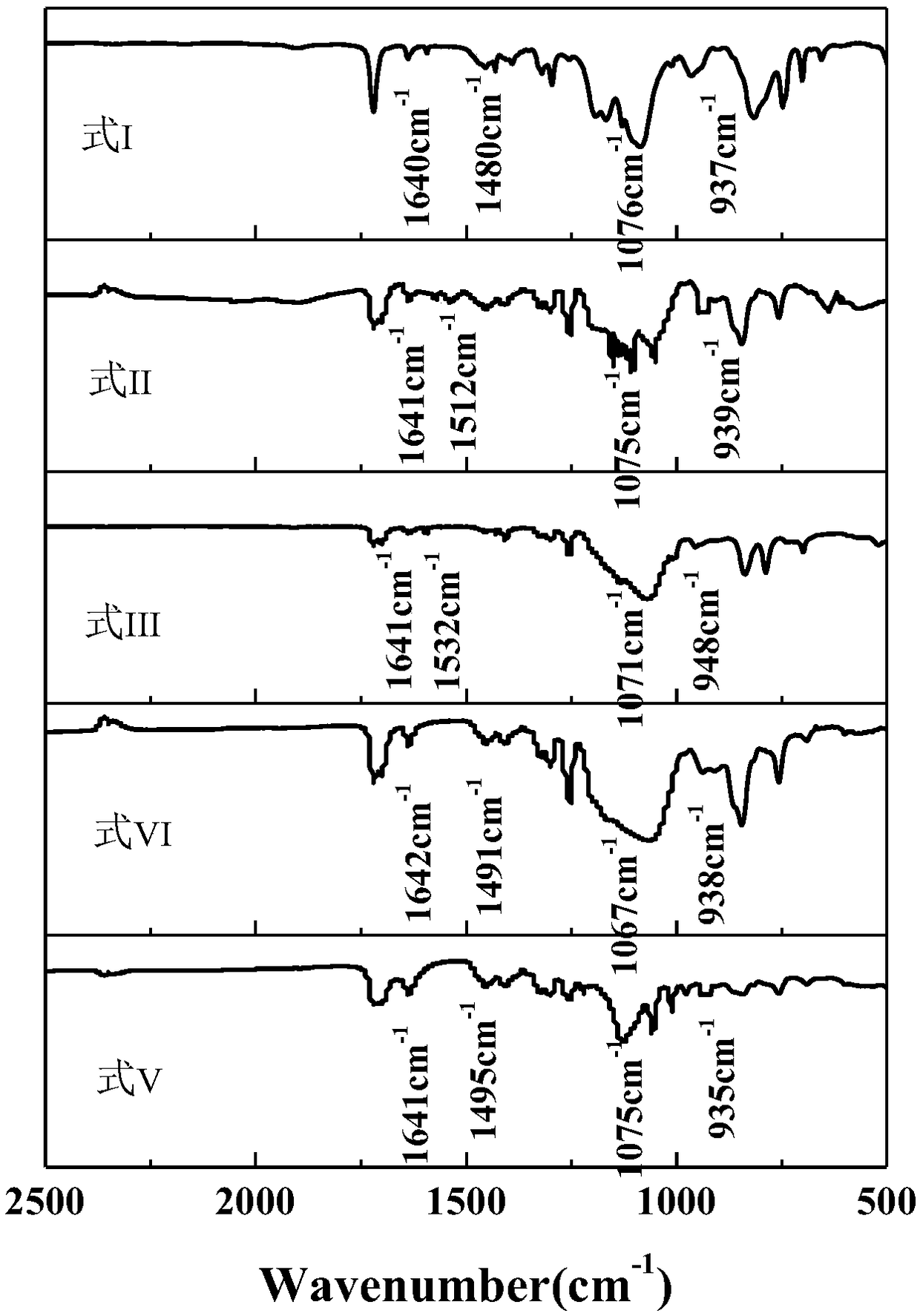
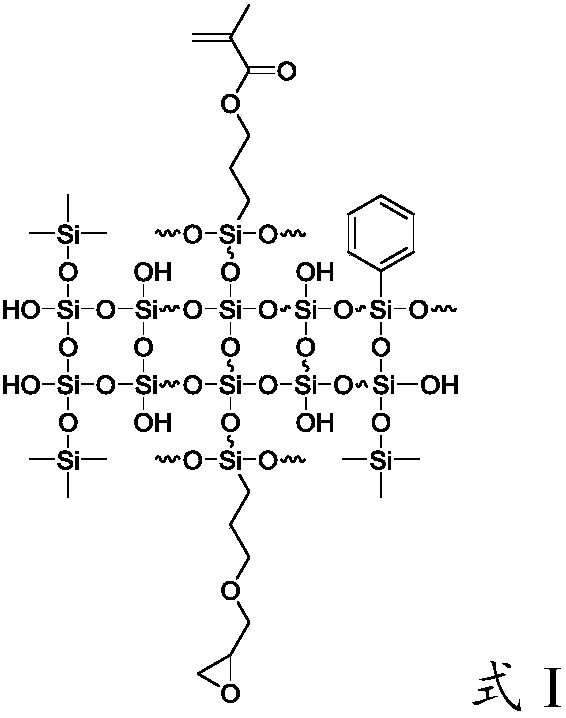
[0053] First add 114g of water, 3.2g of hydrochloric acid and 22g of ethanol into the flask, then add 56.64g of γ-(2,3-epoxypropoxy)propyltrimethoxysilane, 59.66g of γ-methacryloxypropyltrimethoxy Base silane, 53.4g hexamethyldisiloxane and 20.3g phenyltrimethoxysilane were added to the above solution, hydrolysis-polycondensation reaction was carried out at 60°C for 8 hours, and the reaction system was separated to obtain a mixture of silicone resin and water , washed with 30% ethanol aqueous solution for 4 times, and finally vacuumed at 115° C. to remove residual solvent and low boilers to obtain the organosilicon resin mainly having the structure of formula I. Infrared spectroscopy was carried out on it, and the results were shown in figure 1 , figure 1 It is the infrared spectrogram of the photocurable silicone resin prepared in Examples 1-5. figure 1 Among them, formula I is the infrared spectrogram of the photocurable silicone resin prepared in Example 1, formula II is ...
Embodiment 2
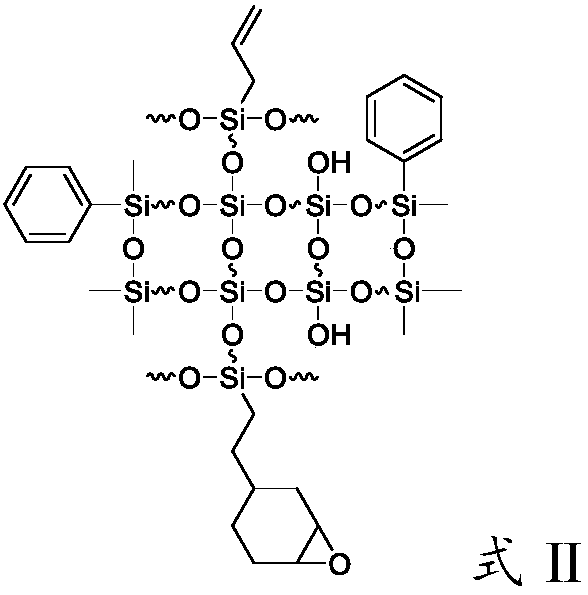
[0058] First 119g of water, 3.7g of potassium hydroxide and 26g of ethanol were added to the flask, then 58.34g of trimethoxy[2-(7-oxabicyclo[4.1.0]hept-3-yl)ethyl]silane, Add 60.25g of allyltriethoxysilane, 60.4g of dimethyldimethoxysilane and 22.7g of phenylmethyldiethoxysilane into the above solution, and conduct a hydrolysis-polycondensation reaction at 70°C for 7 hours. The reaction system was liquid-separated to obtain a mixture of silicone resin and water, washed with 30% ethanol aqueous solution for 3 times, and finally vacuumed at 125° C. to remove residual solvent and low boilers to obtain a silicone resin with the main structure of formula II. Infrared spectroscopy was carried out on it, and the results were shown in figure 1 , figure 1 It is the infrared spectrogram of the photocurable silicone resin prepared in Examples 1-5.
[0059]
[0060] Using a mechanical stirrer, 100g of free radical-cationic photocurable silicone resin, 6.4g of cationic initiator xant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com