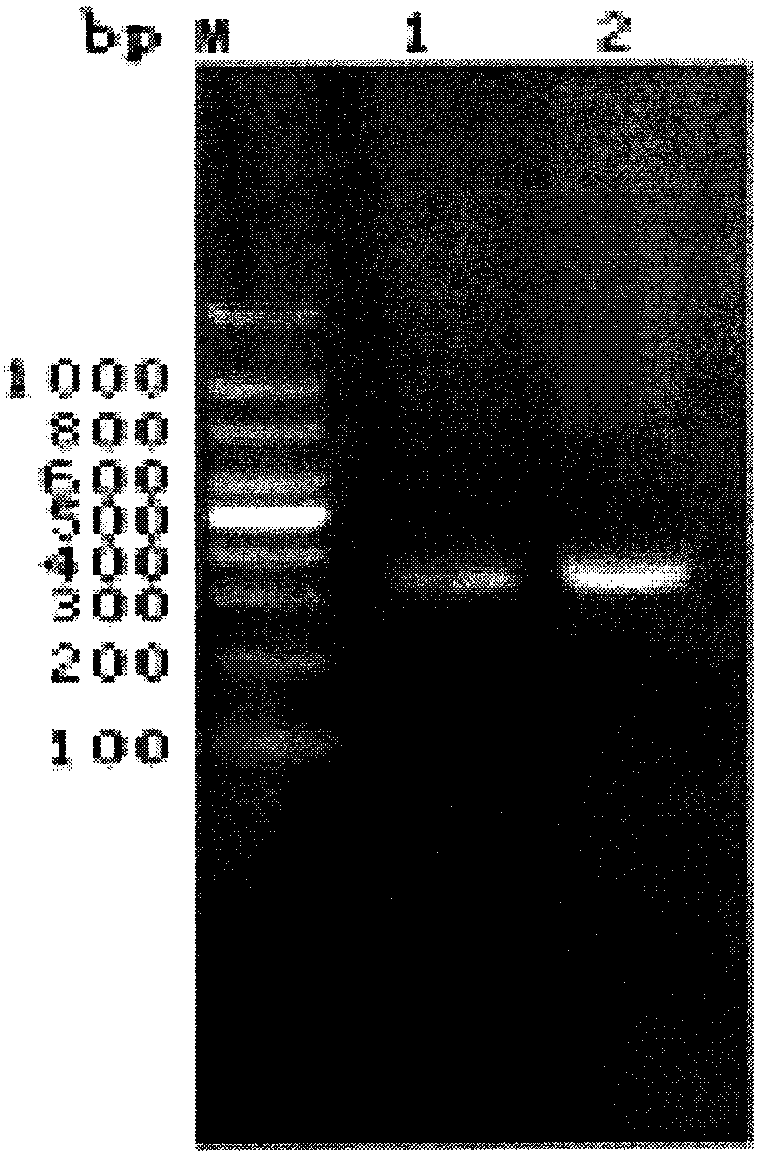Preparation method and application of sclerostin single-chain antibody
A sclerostin and single-chain antibody technology, applied in biochemical equipment and methods, botanical equipment and methods, antibodies, etc., can solve the problem of increasing osteosarcoma, increasing bone density of postmenopausal women, unsatisfactory application of anabolic drugs, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1 Preparation of sclerostin single-chain antibody:
[0067] Sclerostin single-chain antibody needs to be prepared in advance, and then used in the treatment and experimental research of osteoporosis and osteoporosis-related fractures.
[0068] Preparation method of sclerostin single chain antibody:
[0069] Collect the total RNA of SOST monoclonal antibody 5H3D1 hybridoma cells in good growth state, break the cells with T R Izo1 reagent, extract with chloroform, precipitate with isopropanol, wash with 75% ethanol, dry and treat with diethyl pyrocarbonate Water dissolves the RNA. Using the extracted RNA as a template, cDNA was synthesized according to the operating procedures of the RT-PCR kit.
[0070] Design VL and VH primers based on the variable regions of rabbit monoclonal antibodies, and use degenerate codons to adapt to the diversity of light and heavy chains (S=G, C; M=A, C; R=A, G; W=A, T; Y = C, T);
[0071] The Linker is designed on the VL downstre...
Embodiment 2
[0084] Example 2 Analysis of the coding sequence of the sclerostin single-chain antibody:
[0085] The cells produced in Example 1 were purified from the plasmid in the cell line using a DNA extraction kit, and purified and separated by restriction endonuclease cutting and 2% agarose gel electrophoresis to obtain the coding sequence of the heavy chain variable region. The coding sequence of the light chain variable region was obtained by using the method, and the human antibody variable region universal primer was used for polymerase chain reaction amplification.
[0086] The light chain region of sclerostin scFv
[0087] SEQGACCATA TGCTGACCCA GAGTGCATCG CTCGTGTCTG CCGCTGTGCG AAGCATAGTC 60
[0088] ACCATCAATA GCCAGGCCAG TCAGAGTGTT TATAAGAACA ACTACTTAGC CTGGTTTCAG 120
[0089] CAGAAACCAG GGCAGCCTCC CAAGCGCCTG ATCTCATCTG CATCGACTCT GGCTTCTGCG 180
[0090] GTCTCGTCGG GGTTCAACGG CAGTCGATCC GGGACACAGA TCACTCTCAT CATCAGCGAC 240
[0091] GTGCAGTGTG ACGATGCTGG CACTTACTAC TGTCTGGACA ...
Embodiment 3
[0106] Example 3 Application of sclerostin single-chain antibody:
[0107] 1. Detection of the proliferation and differentiation ability of sclerostin single-chain antibody (Scl-scFv) on BMSCs; co-culture of sclerostin single-chain antibody (Scl-scFv) with mouse bone marrow mesenchymal stem cells, and the proliferation of cells was detected by MTT method Ability; BMSCs osteogenic induction 2 days after co-culture with sclerostin single-chain antibody, on the 7th day Real-Time PCR detection type I collagen (COL-1), alkaline phosphatase (ALP), RUNX2, osteopontin OPN, Osteocalcin (OCN) mRNA expression; on the 4th, 7th, and 10th day, Western blot was used to detect type I collagen, and ELISA was used to detect the expression of osteocalcin. Results The OD value of MTT in the Scl-scFv group was not significantly different from that in the blank control group; the expression of type I collagen, alkaline phosphatase, RUNX2, OPN, and OCN mRNA in the experimental group treated with Scl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com