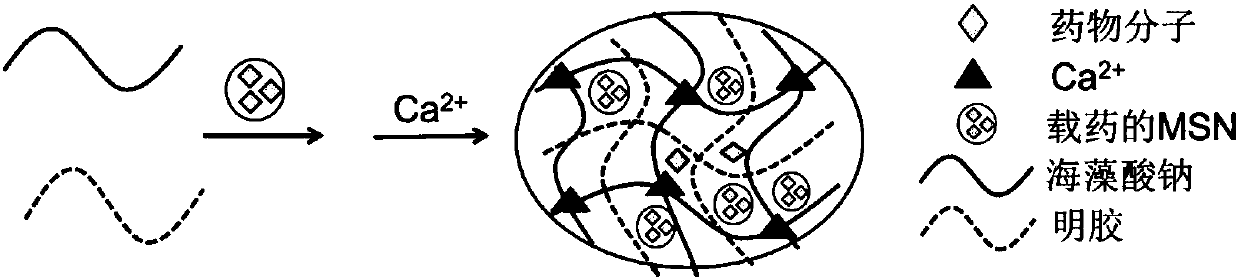
Preparation method and application of nano mesoporous silica doped hydrogel drug controlled-release carrier material
A silica and nano-mesoporous technology, which is applied in drug delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of low bioavailability, poor solubility of IDM, poor fat solubility, etc., and achieve good relief release effect, drug leakage improvement, and mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: A preparation method of a hydrogel drug sustained-release carrier material doped with nanometer mesoporous silica
[0050] Weigh 0.1g of MSN into a volumetric flask filled with 25mL of deionized water, ultrasonically disperse for 1h, and prepare a 0.4% MSN solution. Pipette 10mL of the above MSN solution, add 0.4g gelatin to the solution, first perform limited swelling for 30min, then heat in a water bath at 40°C, stir until the gelatin dissolves, and prepare a 4% gelatin solution. Weigh 0.3 g of sodium alginate powder into another round-bottomed flask containing 10 mL of the above-mentioned MSN solution, stir at room temperature until dissolved, and prepare a 3% sodium alginate solution. Take 10mL of the prepared gelatin solution and 10mL of the prepared sodium alginate solution to prepare a 1:1 mixed solution, stir in a water bath at 40°C, and use a vacuum pump to evenly degas until there are no bubbles, then use the artificial drop method. Slowly drop the...
Embodiment 2
[0052] Example 2: A preparation method of a hydrogel drug sustained-release carrier material doped with nanometer mesoporous silica
[0053] Weigh 0.1g of MSN into a volumetric flask filled with 25mL of deionized water, disperse by ultrasonication for 1h, and prepare a 0.4% MSN solution. Pipette 10mL of MSN solution, add 0.4g of gelatin to the solution, perform limited swelling for 30min, then heat in a water bath at 40°C, stir until the gelatin dissolves, and prepare a 4% gelatin solution. Weigh 0.3 g of sodium alginate powder into another round-bottomed flask containing 10 mL of the above-mentioned MSN solution, stir at room temperature until dissolved, and prepare a 3% sodium alginate solution. Take 10mL of the prepared gelatin solution and 10mL of the prepared sodium alginate solution to prepare a 1:1 mixed solution, stir in a water bath at 40°C, and use a vacuum pump to evenly degas until there are no bubbles, then use the artificial drop method. Slowly drop the mixed so...
Embodiment 3
[0055] Example 3: A preparation method of a slow-release drug-loaded hydrogel
[0056] Take 0.25g BSA, dilute it to 25mL with deionized water, and prepare a 10mg / mL BSA solution; add 0.1g MSN to the BSA solution, disperse ultrasonically until the MSN is completely dispersed, and prepare a 0.4% MSN solution. Take 10mL of the above MSN solution and add 0.4g of gelatin, perform limited swelling for 30 minutes, then heat in a water bath at 40°C, stir until the gelatin dissolves, and prepare a 4% gelatin solution. Take another 10 mL of MSN solution, add 0.3 g of sodium alginate powder, stir and dissolve at room temperature, and prepare a 3% sodium alginate solution. Take 10mL of the prepared gelatin solution and 10mL of the prepared sodium alginate solution to form a 1:1 mixed solution, stir in a water bath at 40°C, and degas evenly with a vacuum pump, then use the manual drop method to slowly dissolve the mixed solution at room temperature. Add dropwise 400mL of 2% CaCl 2 In the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com