Gel polypeptide capable of being used for preparing hemostatic material
A technology of hemostatic materials and products, applied in the direction of medical preparations containing active ingredients, peptides, blood diseases, etc., can solve the problems of easy cracking and falling off, poor hemostatic effect, rebleeding, etc., and achieve good biocompatibility, The effect of good coagulation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: Design and synthesis of polypeptide
[0022] The applicant has designed and screened ion-complementary short peptides, so that the screened peptides have the driving force and guiding effect of self-assembly. The driving force of self-assembly refers to the synergistic effect of non-covalent bond forces between molecules, which provides energy backing for the self-assembly of peptide molecules; while the guiding effect of self-assembly refers to the geometric complementarity of the spatial structure of peptide molecules , only when the spatial structure of peptide molecules is complementary, can they be stacked and rearranged in spatial size and direction.
[0023] In order to realize the self-assembly effect of the designed polypeptide combined with the blood, the applicant designed the self-assembling peptide to contain acidic amino acid residues and basic amino acid residues as polar amino acids, and the insertion position and quantity of non-polar amin...
Embodiment 2
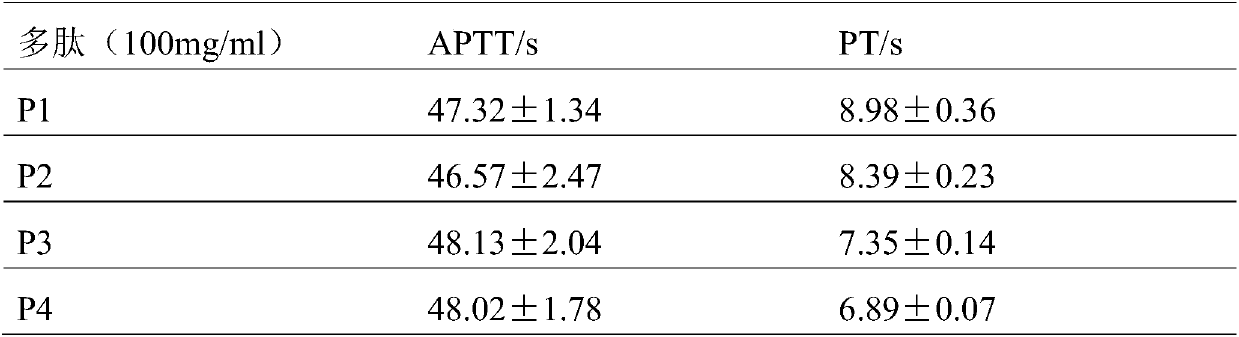
[0030] Example 2: Determination of coagulation activity
[0031] In order to evaluate the in vitro coagulation performance of the designed polypeptide, the activated partial thromboplastin time (APTT) and prothrombin time (PT) were used for analytical tests: activated partial thromboplastin time (APTT) and prothrombin time (PT) ) was determined using a commercial kit.
[0032] Blood sample preparation:
[0033] First, SD rats were anesthetized with pentobarbital sodium solution (0.3 μl / 100mg), blood was drawn from the rat femoral artery, and then it was anticoagulated by 3.8% sodium citrate anticoagulant, at 37 ° C, Platelet-poor plasma was obtained by centrifugation at 3000r / min.
[0034] Preparation of peptide solution:
[0035] Precisely weighed short peptide freeze-dried powder was added to ultrapure water to prepare a mother solution with a concentration of 500mg / ml, sealed and stored in a refrigerator at 4°C in the dark for later use, and diluted to a corresponding co...
Embodiment 3
[0043] Carry out atomic force microscope (AFM) detection to the polypeptide whose amino acid sequence is SEQ ID NO:4:
[0044] Place 20 μl of the peptide solution on the newly dissociated mica sheet, let it stand for 15 s, and then wash 3 times along the edge of the droplet with 100 μl of ultrapure water. Stand at room temperature at 30°C, and observe with AFM after the mica sheet dries naturally. The AFM parameters are set to tapping mode, the scanning area is 2μm×2μm, and the scanning frequency is 1.0Hz; the scanning probe (Model: Arrow UHFAuD) parameters: the elastic coefficient (k) is 6 (1.5~21.0) N / m, and the material is Si , uncoated, with a tip radius of (10±2) nm.
[0045] Carry out transmission electron microscopy (TEM) detection to the polypeptide whose amino acid sequence is SEQ ID NO:4:
[0046] Drop 30 μl of peptide solution on the parafilm, then place a 300-mesh carbon-coated copper grid on the sample for about 30 seconds, take out the copper grid with clean tw...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com