A doxorubicin precursor compound with photoresponse degradation and its preparation and application
The technology of a precursor compound, doxorubicin, is applied to the preparation of sugar derivatives, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problem of incomplete release of doxorubicin preparations Unable to time, fixed-point release, toxic and side effects and other problems, to achieve the effect of large drug load, small volume and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the preparation of prodrug intermediate 1 formula (II) compound
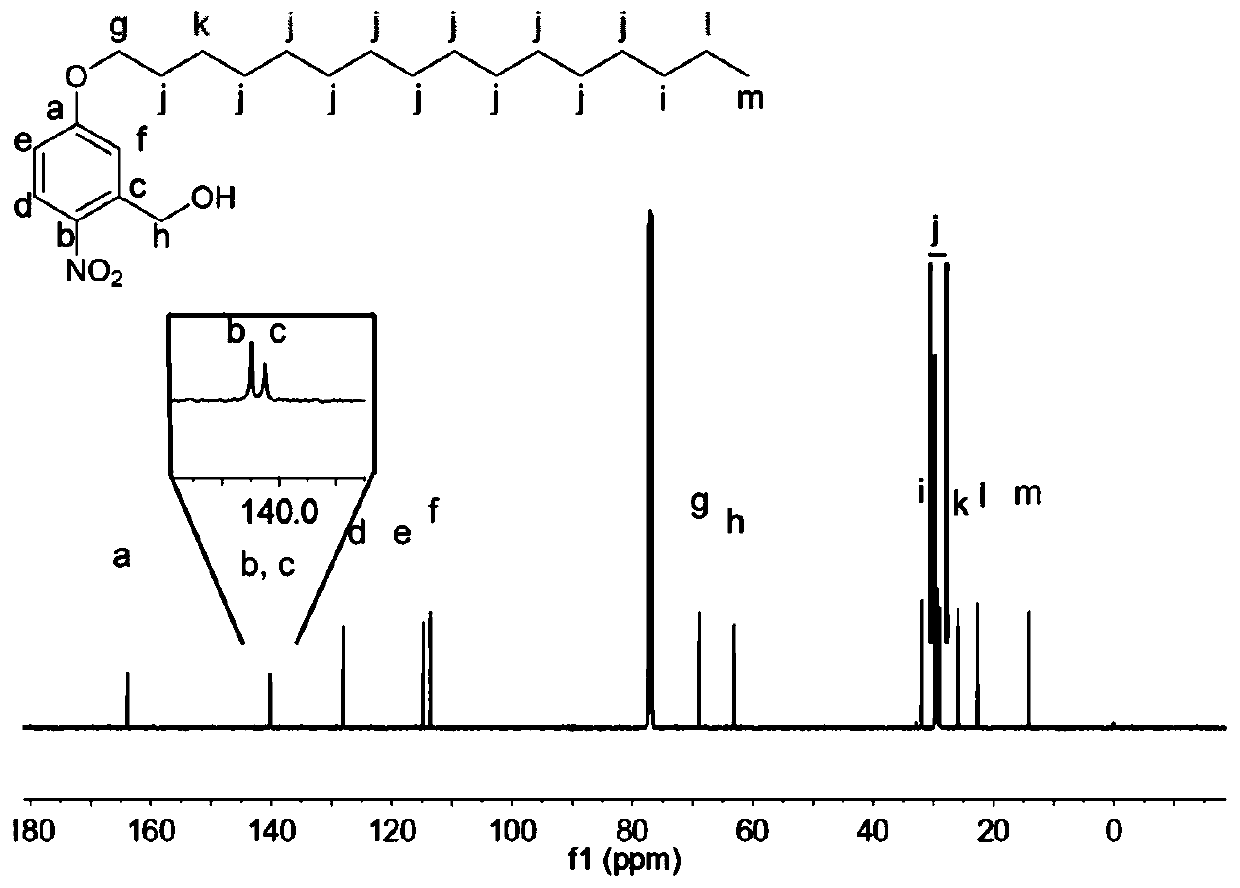
[0041] Dissolve 340mg (2mmol) of 5-hydroxy-2-nitrobenzyl ethanol, 733mg (2.4mmol) of hexadecane bromide in 20mL of DMF, add 424mg (4mmol) of NaCO 3 , 80°C for 24 hours. After the reaction, the DMF was distilled off under reduced pressure, and the crude product was vigorously stirred overnight with a mixed solvent of 50mL water and 50mL ethyl acetate. Anhydrous Na 2 SO 4 Let dry overnight. After filtration and concentration of the filtrate under reduced pressure, the product was separated by silica gel column chromatography to obtain a light yellow solid product. The eluent was ethyl acetate:petroleum ether, and the yield was 83%.
[0042] Intermediate 1 The structure of the compound of formula (II) was characterized by the results of NMR and C NMR, and the results are as follows figure 1 and figure 2 shown.
Embodiment 2
[0043] Embodiment 2: the preparation of prodrug intermediate 2 formula (Ⅲ) compound
[0044] Phenyl p-nitrochloroformate (148 mg, 0.73 mmol) was dissolved in 2 mL of tetrahydrofuran, and 148 mg (0.7 mmol) of intermediate 1 and 256 μL (1.47 mmol) of N,N-diisopropylethylamine were added to 2 mL of tris Chloromethane solution, reacted for 24 hours. Additional phenyl p-nitrochloroformate (148 mg, 0.73 mmol) and 4-dimethylaminopyridine (90 mg, 0.74 mmol) were added, and the reaction was continued for 4 hours. The solvent was removed under reduced pressure, 50 mL of ethyl acetate was added and dissolved in 50 mL of 1M H 3 PO 4 and saturated NaHCO 3 Washed twice successively, the organic layer was washed with anhydrous Na 2 SO 4 Let dry overnight. After filtration and concentration of the filtrate under reduced pressure, the product was separated by silica gel column chromatography to obtain a light yellow solid product. The eluent was ethyl acetate:petroleum ether, and the yie...
Embodiment 3
[0046] Embodiment 3: the preparation of doxorubicin precursor compound (DOC)
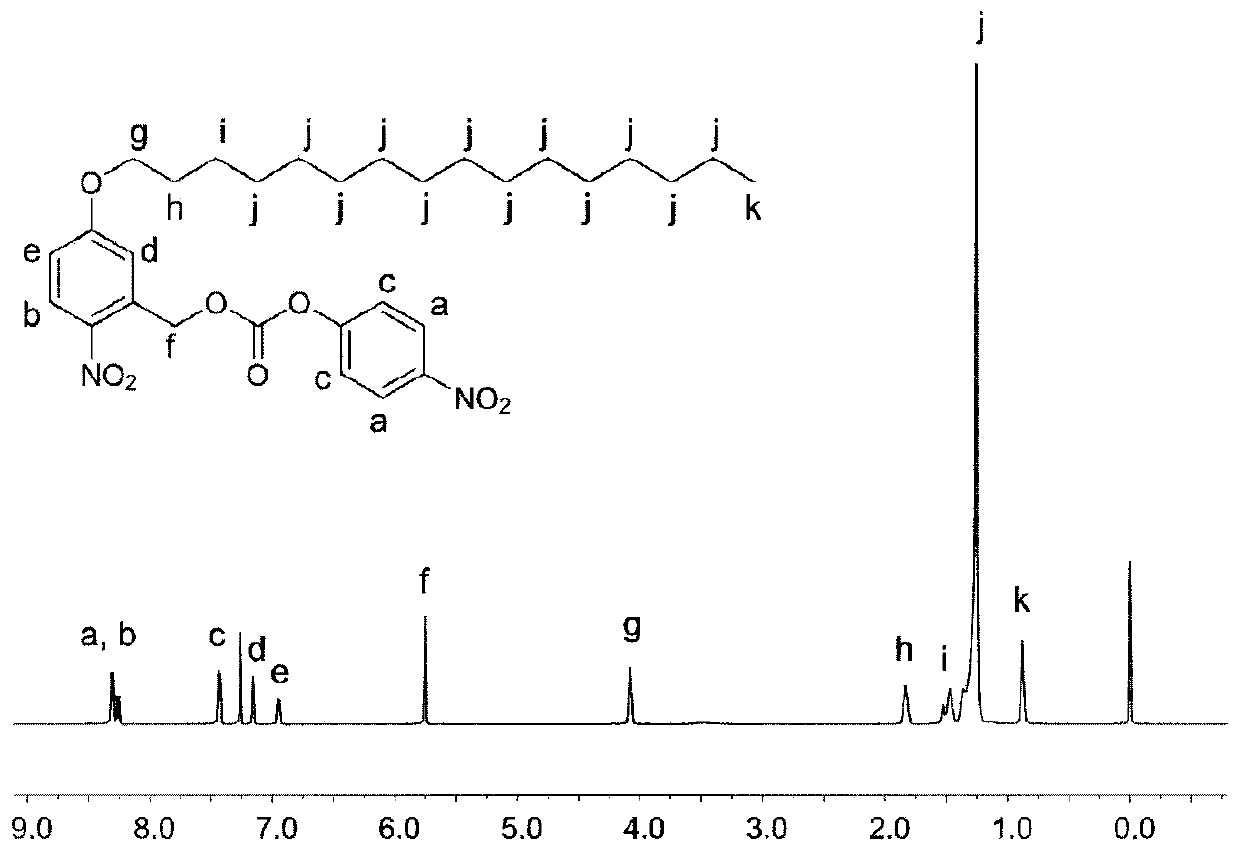
[0047]Doxorubicin hydrochloride (50 mg, 86.2 μmol) was dissolved in 6 mL of DMF, triethylamine (36 μL, 258 μmol) and intermediate 2 (49 mg, 86.8 μmol) were added. After reacting at room temperature in the dark for 36 hours, the solvent was removed under reduced pressure, and the residue was dissolved in 4 mL of a mixed solvent of 3% methanol / chloroform, separated by a silica gel column, and finally the product was obtained.
[0048] The structure of doxorubicin precursor compound was characterized by H NMR and C NMR results, the results are as follows Figure 5 and Figure 6 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com