ATP responding type drug-releasing nano gel and preparation method thereof
A nanogel and drug technology, applied in the field of medicine, can solve the problems of normal tissue side effects and poor targeting of drug delivery systems, and achieve the effects of improving safety and targeting, reducing toxic and side effects, and improving therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0025] step 1:
[0026] Send the following 3 DNA oligonucleotide strands to the company for synthesis:
[0027] ATP aptamer: 5'-ACC TGG GGG AGT ATT GCG GAG GAA GGT-3'
[0028] DNA1: 5'-CTC TCT CTC TTT ACC TTC CTC CGC-3'
[0029] DNA2: 5'-ACT CCC CCA GGT AAA GAG AGA GAG-3'
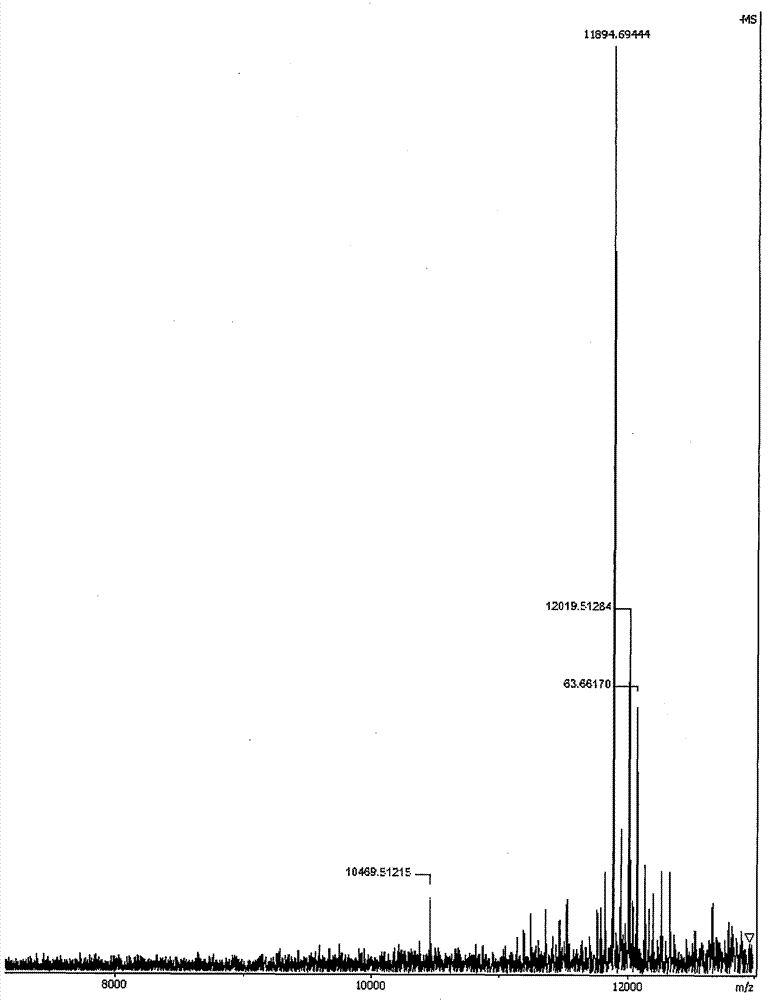
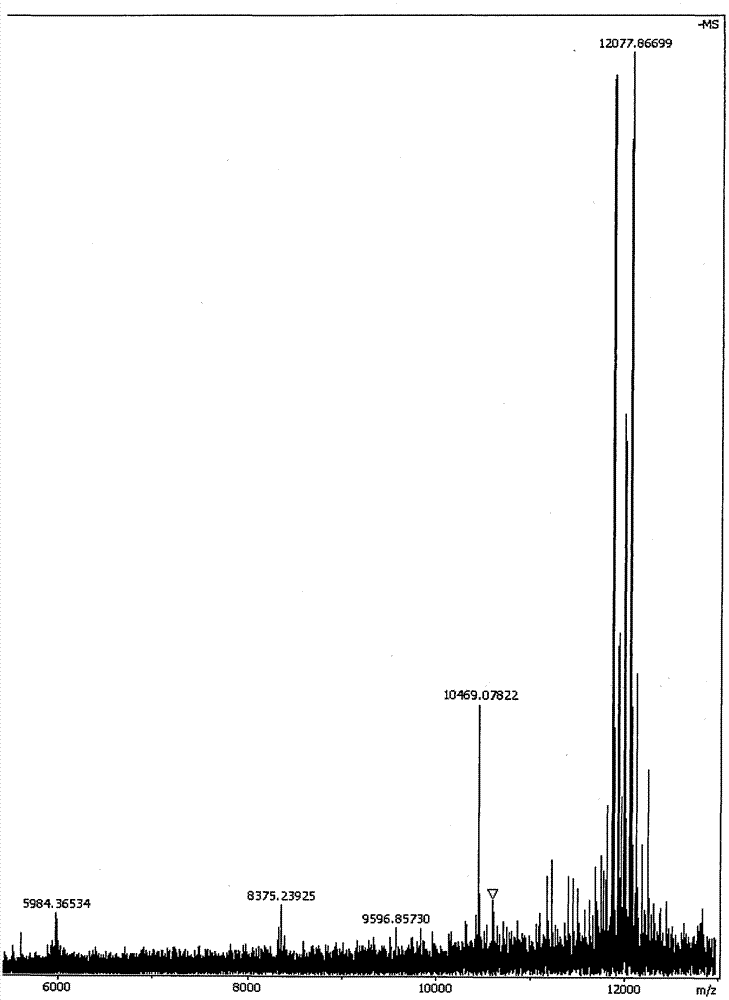
[0030]Dissolve the single strands of DNA1 (100.76nmol) and DNA2 (71.86nmol) in 1mL of MES buffer (0.1M, pH4.9), and then add 1-ethyl-(3-dimethylaminopropyl)carbodiene Amine hydrochloride (1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride, EDC.HCL) and N-hydroxysuccinimide (N-hydroxysuccinimide, NHS), activated at room temperature for 2h, adding a certain amount of carboxymethyl Based on chitosan, shake at 1200rpm at room temperature for 24h, rinse with MES buffer (0.1M, pH 4.9), add 1mL of ammonia water (28%), and react for 24h at 40°C; take the supernatant and use a dialysis bag (MWCO: 1000 ) dialyzed for 16 hours; freeze-dried to obtain white flocculent powder CMCS-DNA1 and CMCS-DNA2. The s...
specific Embodiment 2
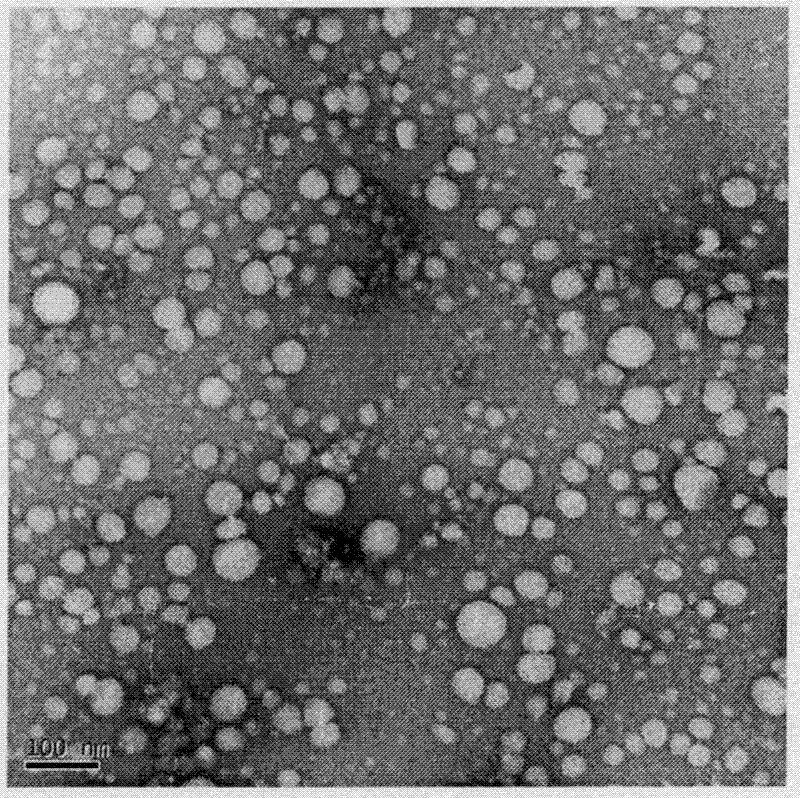
[0032] The ATP-responsive drug-releasing nanogel obtained in Example 1 of the present invention is investigated for ATP-responsive drug release, see attached image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com