Glutamine synthetase gene with glufosinate tolerance and application thereof
A glufosinate-ammonium resistance technology, applied in the field of genetic engineering, can solve problems such as plant death, amino acid synthesis and photosynthetic chlorophyll destruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Screening of natural bacterial strains producing glutamine synthetase
[0021] (1) Screening of natural strains producing glutamine synthetase:
[0022] The applicant's laboratory isolated nearly 200 strains of marine bacteria from seawater near Qingdao, Shandong. The marine strains preserved by the State Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University, where the applicant is located, were placed in high-salt LB (1% peptone, 0.5% yeast Culture and 2% NaCl, pH7.0) after activation on the plate, single bacterial colonies were picked out and cultured in liquid high-salt LB medium, and after cultivating at 28°C for 36 hours, the cells were broken by ultrasonic waves (30HZ, 400W), respectively. The supernatant after fermentation and the supernatant after cell disruption were used to measure glutamine synthetase enzyme activity (the enzyme activity assay method is as follows). Through experiments, a bacterial strain with high glutamin...
Embodiment 2
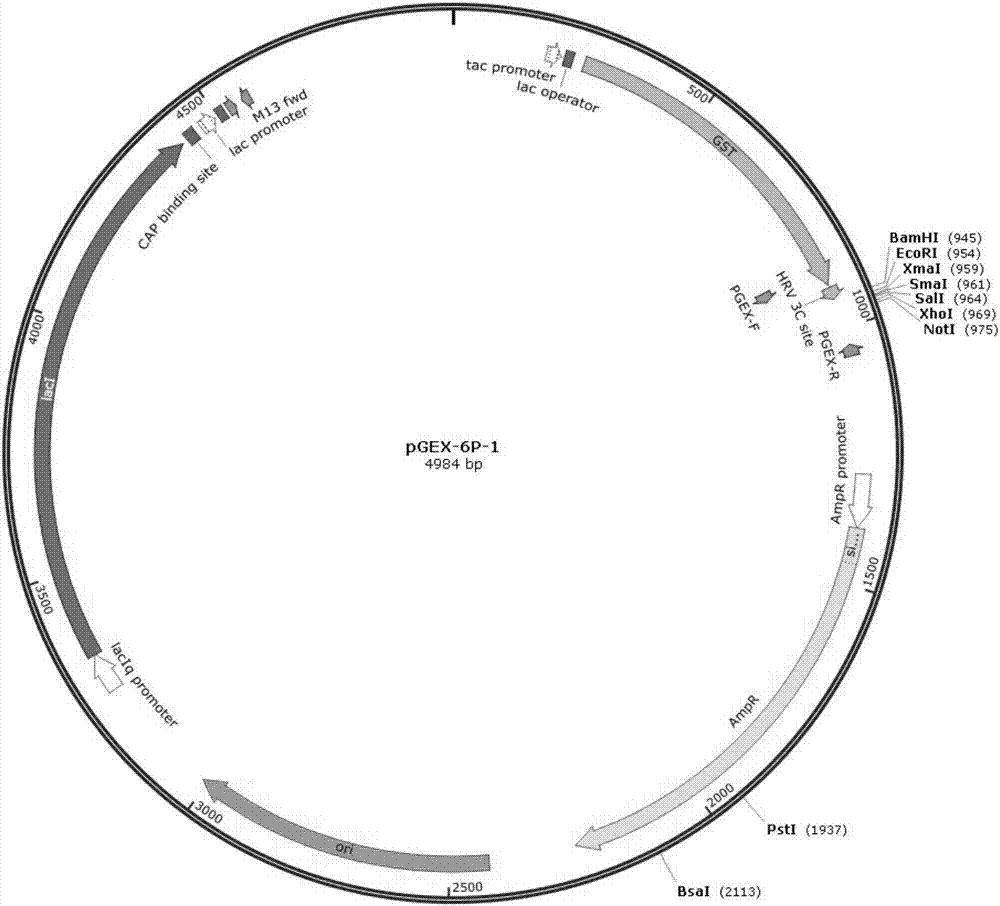
[0037] Example 2: Cloning, expression and purification of exiGS gene in Escherichia coli
[0038] The specific methods and steps are as follows:
[0039] 1. Extract (Exiguobacterium sp) chromosomal DNA:
[0040] (1) After culturing the isolated Exiguobacterium sp. strain in a high-salt [sodium chloride content of 100 μl 5mol / L, see step (4)] LB liquid culture at 28°C for 24 hours, take 1.5ml of the bacterial liquid and sterilize it once Centrifuge at 12,000 rpm for 1 minute, discard the supernatant, and collect the bacteria.
[0041] (2) Wash the bacteria twice with TE buffer (50mmol / LTris-HCl pH8.0, 10mmol / LEDTA pH8.0), then add 50μl 100μg / ml lysozyme (purchased from sigma company) to suspend the bacteria. 37°C water bath for one hour.
[0042] (3) Add 520 μl TE buffer, 30 μl 10% SDS and 3 μl 20 mg / ml proteinase K (purchased from sigma company) and mix well, then place in a water bath at 37° C. for one hour.
[0043] (4) Add 100 μl of 5 mol / L sodium chloride solution and ...
Embodiment 3
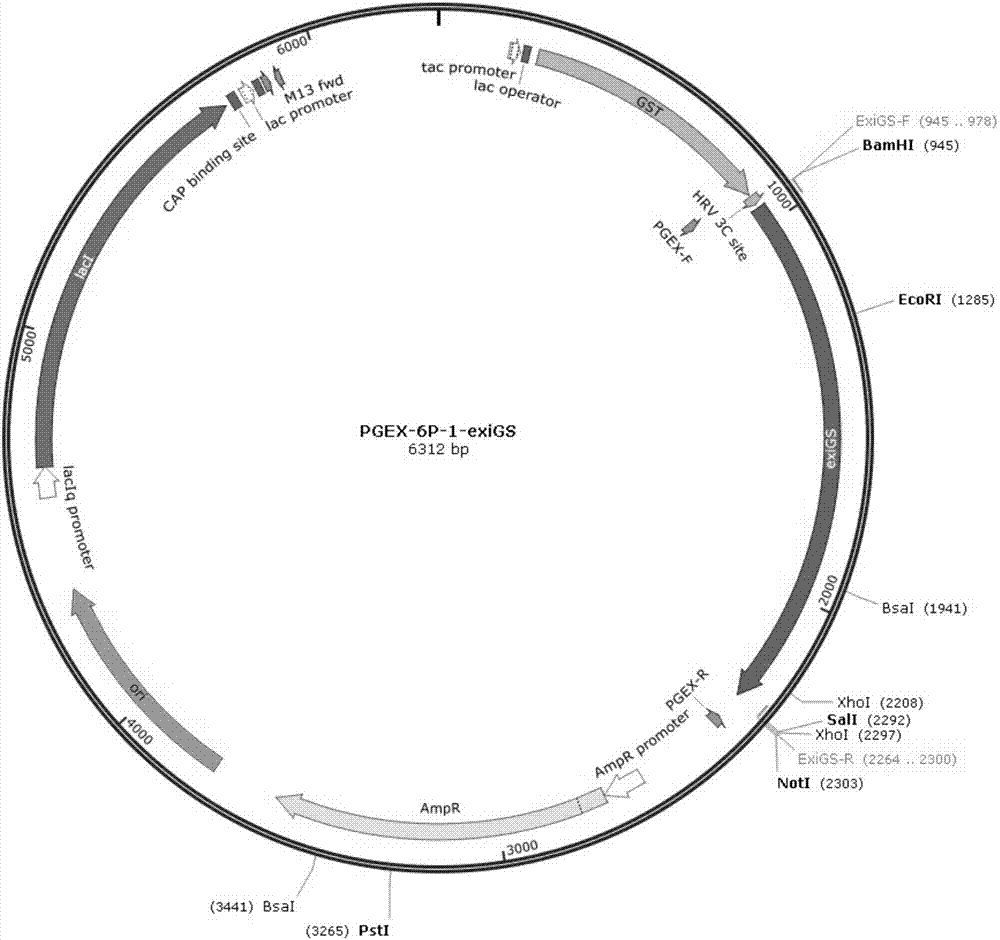
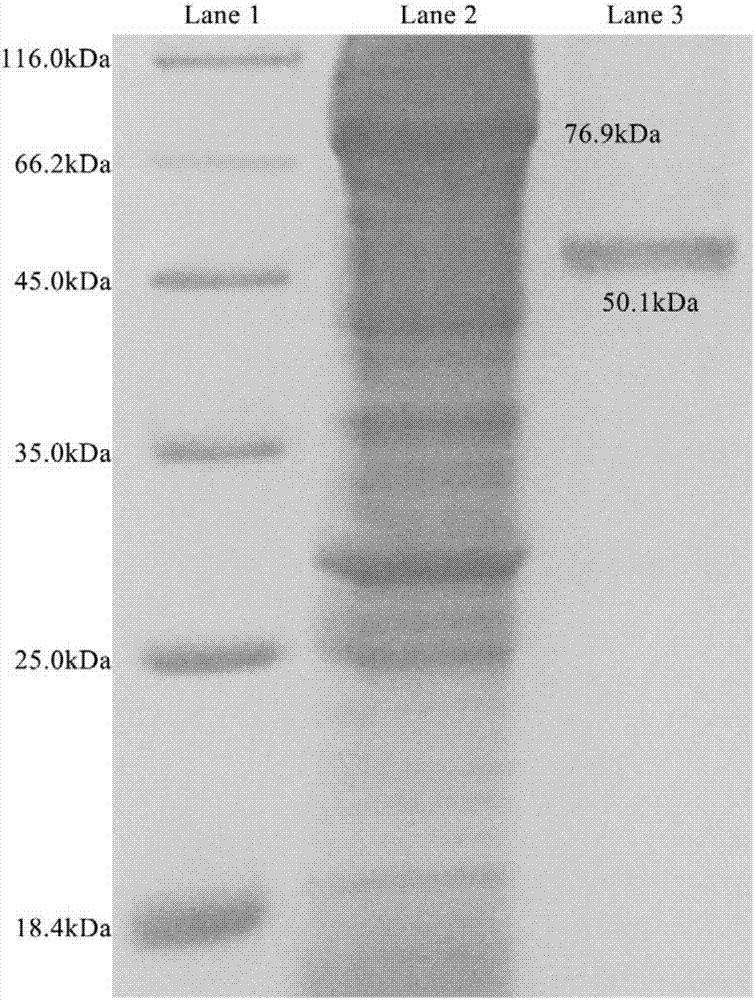
[0069] Embodiment 3: Expression and purification of glutamine synthetase:
[0070] 1. Induced expression of recombinant glutamine synthetase in Escherichia coli:
[0071] The recombinant plasmid pGEX-6P-exiGS was transformed to express purified glutamine synthetase in Escherichia coli BL21(DE3). Mix the plasmid pGEX-6P-exiGS with 100 μl of Escherichia coli competent cells BL21 (DE3), place in ice bath for 30 minutes, treat at 42°C for 90 seconds, add 800 μl of LB medium (1% tryptone, 1% Nacl, 0.5% Yeast powder, pH 7.0), incubated at 37°C for 1 hour, spread a solid LB plate containing 100 μg / ml ampicillin, incubated at 37°C for 14 hours, and picked transformants to induce the expression of glutamine synthetase.
[0072] Transfer overnight activated transformants to 1L LB liquid medium containing 100μg / ml ampicillin at 1% inoculum size, culture at 37°C for 2-3hrs, and make the OD 600 When it reaches 0.6-0.7, add IPTG to a final concentration of 0.2mM, and culture at 18°C at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com