Ceftriaxone sodium and sulbactam sodium composition as well as medicinal preparation containing composition and application thereof
A technology for ceftriaxone sodium and sulbactam sodium, which is applied to pharmaceutical preparations and their application fields, can solve the problems of short validity period, poor efficacy, poor stability and the like, and achieve the effects of excellent stability and biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Get ceftriaxone sodium 1200g and sulbactam sodium 600g prepared according to the method of comparative example 2, grind together to particle diameter D with ball ink machine 5025 μm ~ 47 μm, and then placed in a conical ribbon mixer (HF1600 type) to mix evenly, the machine speed is set at 30 revolutions per minute, and the mixing time is 25 minutes. After the operation was completed, the materials were taken out to obtain a crystalline substance, which was named Composition I.
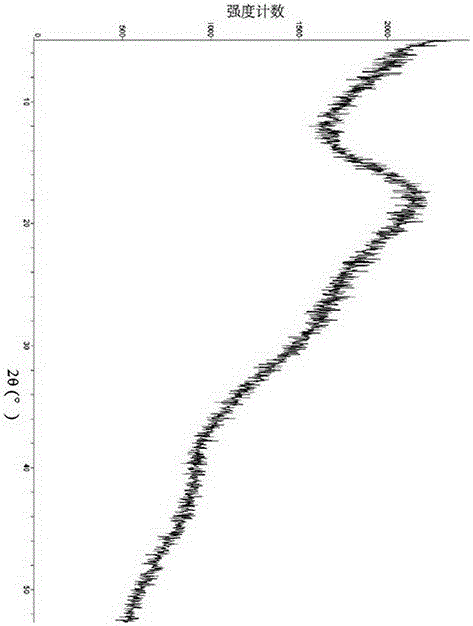
[0088] Sampling was taken to determine its crystal form, and the X-ray powder diffraction pattern was shown in image 3 , Table 1 lists the main data in the spectrum.
[0089] Its infrared absorption is measured by infrared absorption spectrum analysis, and the spectrum is shown in Figure 4 , Table 2 lists the main infrared absorption peak data.
[0090] Differential scanning calorimetry was performed and an exothermic peak was found at 269.51°C.
[0091] serial number 2θ angle ...
Embodiment 2
[0094] Get 500g of commercially available ceftriaxone sodium and 500g of sulbactam sodium, grind together to particle size D with a ball ink machine 50 It is 38μm~62μm. Then place it in a single-cone ribbon mixer (HF1600 type) and mix evenly, the machine speed is set at 40 revolutions per minute, and the mixing time is 20 minutes. After the operation was completed, the material was taken out to obtain a crystalline substance, which was named Composition II.
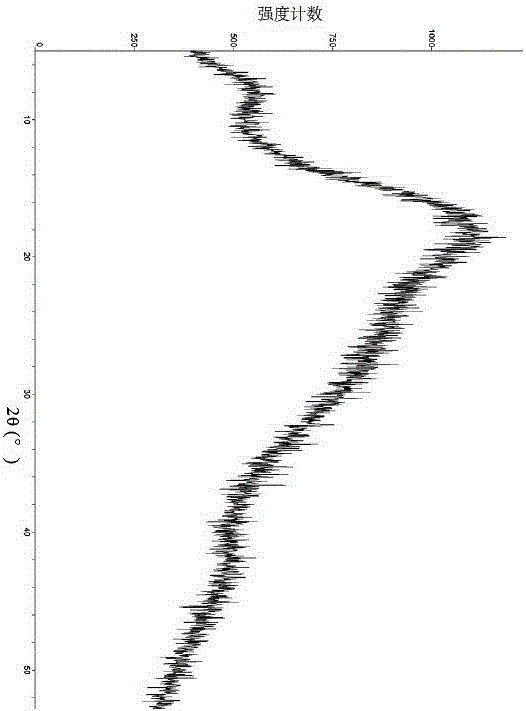
[0095] Sampling was taken to determine its crystal form, and the X-ray powder diffraction pattern was shown in Figure 5 , Table 3 lists the main data in the map.
[0096] Its infrared absorption is measured by infrared absorption spectrum analysis, and the spectrum is shown in Image 6 , Table 4 lists the main infrared absorption peak data.
[0097] Differential scanning calorimetry was performed and an exothermic peak was found at 269.67°C.
[0098] serial number 2θ angle (°) Interplanar spacing (Å) ...
Embodiment 3
[0101] Get 930g of commercially available ceftriaxone sodium and grind it; get 310g of sulbactam sodium and grind it, and then grind the two kinds of raw materials together to a particle size of D 50 It is 58μm~88μm. Place in a single-cone ribbon mixer (HF1600 type) and mix evenly, the machine speed is set at 25 revolutions per minute, and the mixing time is 10 minutes. After the operation was completed, the material was taken out, and the obtained crystalline material was named Composition III.
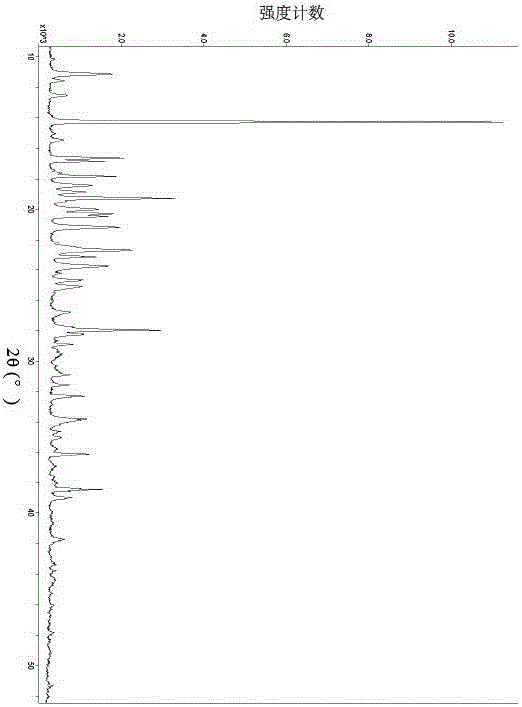
[0102] Sampling was taken to determine its crystal form, and the X-ray diffraction pattern was shown in Figure 7 , Table 5 lists the main data in the map.
[0103] Its infrared absorption is measured by infrared absorption spectrum analysis, and the spectrum is shown in Figure 8 , Table 6 lists the main infrared absorption peak data.
[0104] Differential scanning calorimetry was performed and an exothermic peak was found at 269.69°C.
[0105] serial number 2θ angl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com