DHAV (Duck Hepatitis A Virus) III type complex live vaccine and preparation method thereof
A duck hepatitis A virus and complex technology, applied in biochemical equipment and methods, vaccines, viruses, etc., can solve the problems of time-consuming, labor-intensive, expensive vaccines, and high production costs, and achieve a wide safety range, good safety, and good protection. effect of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
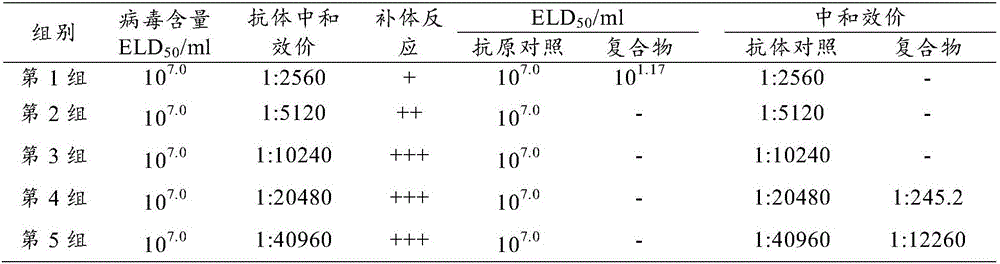
[0027] Example 1 Virus and egg yolk antibody ratio screening and detection test
[0028] 1. Preparation of virus stock solution
[0029] 1.1 Inoculation
[0030] Duck hepatitis A virus type III JS57 strain (microorganism preservation number is: CGMCC No.9708) is made 1000-fold dilution with sterilized normal saline, inoculates 0.2ml in every embryo allantoic cavity of 9-day-old SPF chicken embryo, seals pinhole after inoculation, Continue incubation at 37°C without turning the eggs.
[0031] 1.2 Incubation and observation
[0032] After 24 hours of inoculation, the eggs were illuminated every 4 hours until 96 hours. Take chicken embryos that died within 24 to 96 hours and cool them at 2 to 8°C.
[0033] 1.3 harvest
[0034] Chick embryos that have been cooled for 4 to 24 hours are collected by aseptic surgery, including chorioallantoic membrane, fetus, embryo fluid and amniotic fluid, and each of them is mixed into a group and placed in a sterilized bottle. While harvest...
Embodiment 2
[0072] Example 2 Preparation of duck hepatitis A virus type III complex live vaccine
[0073] 1. Preparation of virus stock solution
[0074] 1.1. Preparation of poisonous seeds for production
[0075] Take 9-day-old SPF chicken embryos, inoculate the basic virus species (that is, duck hepatitis A virus type Ⅲ chicken embryo attenuated strain JS57 strain (microorganism preservation number: CGMCC No.9708)), incubate at 37°C, and irradiate the embryos 2 to 4 times a day , Observed for 4 days, discarded dead chicken embryos within 24 hours, and collected allantoic fluid and embryo bodies from chicken embryos that died within 96 hours for sterility testing, and observed fetal lesions. Freezing and thawing 3 times, centrifuging at 4,000rpm for 30min, absorbing the supernatant, quantitatively dispensing into glass bottles, storing in a -80°C refrigerator, and inspecting according to the inspection standards specified by the production seed batch, to obtain the production poisonous ...
Embodiment 3
[0122] Freeze and vacuum dry immediately after subpackaging, according to page 437 of the appendix of the 2000 edition of the "Procedures". Example 3 Duck Hepatitis A Virus Type III Complex Live Vaccine Finished Product Inspection
[0123] The finished product of duck hepatitis A virus type III complex live vaccine prepared in Example 2 of the present invention was tested.
[0124] 1. Physical properties
[0125] It is a light red spongy loose mass, easy to separate from the bottle wall, and dissolves quickly after adding diluent.
[0126] 2. Sterility test
[0127] Tested according to the appendix of "Chinese Veterinary Pharmacopoeia", it should grow aseptically.
[0128] 3. Mycoplasma test
[0129] Tested according to the appendix of "Chinese Veterinary Pharmacopoeia", there should be no mycoplasma growth.
[0130] 4. Exogenous virus inspection
[0131] Test according to the appendix of "Chinese Veterinary Pharmacopoeia", there should be no exogenous virus contaminatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com