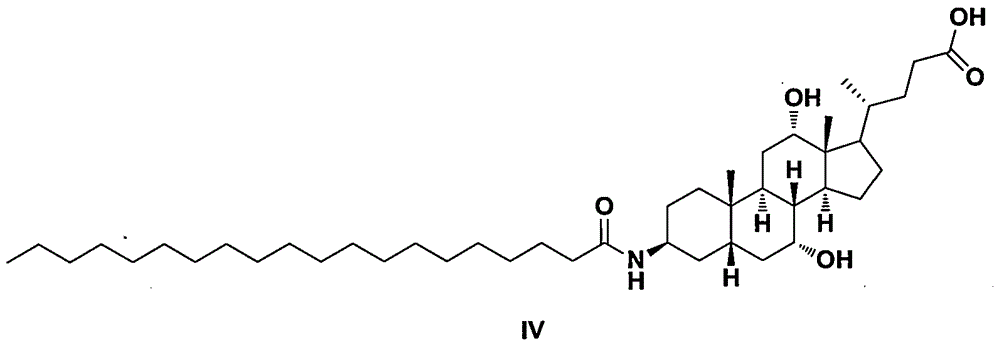
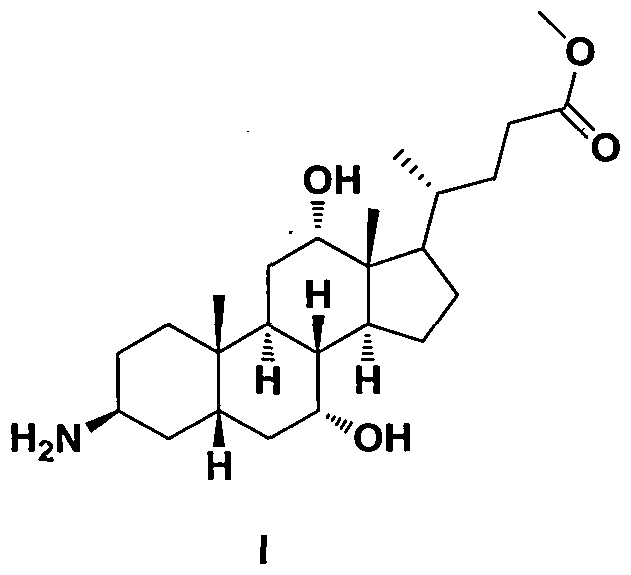
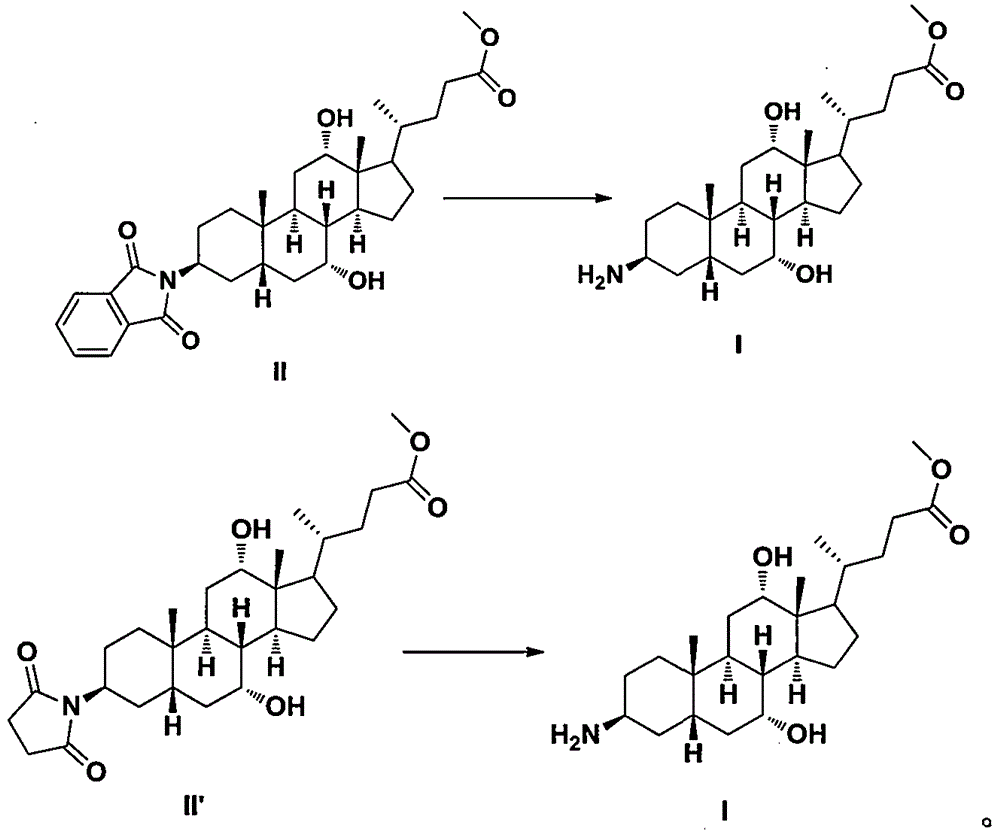
Preparation methods of arachidic cholic acid and arachidic cholic acid intermediate
A technique for intermediates and cholic acid, applied in the field of preparation of arachidonic cholic acid and intermediates thereof, can solve the problems of high production cost, harsh reaction conditions, unsuitable for industrialized production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075]
[0076] Dissolve 1200g of methyl cholate intermediate III in 6800mL of tetrahydrofuran, cool to 0-5°C, add 590g of diethyl azodicarboxylate, and then add 430g of phthalimide. Add 800 g of triphenylphosphine in batches under stirring, stir at 10-20° C. for 16 hours after the addition, add 10 L of water, concentrate under reduced pressure to remove tetrahydrofuran, extract three times with 5 L of dichloromethane, and use 5% sodium sulfite (described The mass concentration is meant that the quality of sodium sulfite accounts for the percentage of the total mass of sodium sulfite aqueous solution), mass concentration 7% sodium bicarbonate (the mass concentration refers to the percentage that the quality of sodium bicarbonate accounts for the total mass of sodium bicarbonate aqueous solution), mass concentration 10% salt water (the mass concentration refers to the percentage of the mass of sodium chloride in the total mass of salt water) was washed twice, dried with anhyd...
Embodiment 2
[0078]
[0079] Dissolve 60 g of methyl cholate intermediate III in 470 mL of isopropyl ether, cool to 0-5°C, add 34 g of diisopropyl azodicarboxylate, and then add 17 g of succinimide. Add 22g of tricyclohexylphosphine in batches under stirring, stir at 10°C to 20°C for 16 hours after the addition, add 500mL of water, concentrate under reduced pressure to remove isopropyl ether, extract three times with 250mL of dichloromethane, and use a mass concentration of 5% Sodium sulfite (the mass concentration refers to the percentage that the quality of sodium sulfite accounts for the total mass of sodium bicarbonate aqueous solution), mass concentration 7% sodium bicarbonate (the mass concentration refers to the percentage that the quality of sodium bicarbonate accounts for the total mass of sodium bicarbonate aqueous solution ), mass concentration 10% salt water (the mass concentration refers to the percentage of the quality of sodium chloride in the total mass of salt water), wa...
Embodiment 3
[0081]
[0082] With peanut cholic acid intermediate II 1250g (purity 97.71%, differential refractive index detector detection), be dissolved in tetrahydrofuran 2500mL and methanol 2500mL, then add mass concentration and be 40% hydrazine hydrate 620mL (the described mass concentration refers to the quality of hydrazine % of the total mass of hydrazine hydrate), after the addition, stir at 15°C to 25°C for 8 hours, add 5L of water, concentrate under reduced pressure to remove tetrahydrofuran and methanol, extract twice with 4L of dichloromethane, and the mass concentration is 10% saline (The mass concentration refers to the percentage of the mass of sodium chloride in the total mass of the saline) washed twice, dried with anhydrous sodium sulfate and then concentrated to obtain a light yellow solid. Then recrystallized with 3.9 L of ethyl acetate to obtain 875 g of arachidic cholic acid intermediate I as off-white solid, with a yield of 91.6% and a purity of 98.78% (detected ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com