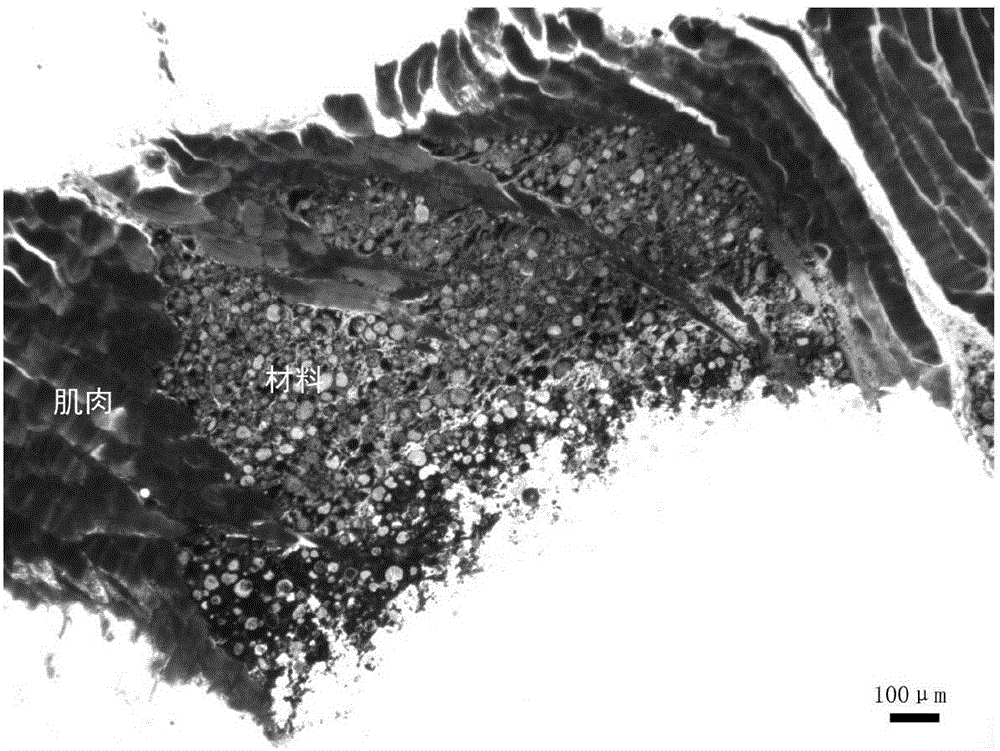
Injectable calcium phosphate/natural polymer composite material and preparation method and application thereof
A technology of natural polymers and composite materials, applied in the field of biomedical materials, can solve the problems of returning to the original state within 5-1 years, producing granulomas, difficult plastic effects, etc., and achieving good clinical application prospects and good shaping effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 Preparation of injectable calcium phosphate / sodium hyaluronate composite material of the present invention
[0026] 1. Preparation method
[0027] 1) Prepared with sodium hyaluronate (molecular weight 1000kDa, Shandong Huaxi Freda Biomedical Co., Ltd.) modified by 1,4-butanediol glycidyl ether (BDDE) (modification degree 6-10%) into 10ml of 2.5% aqueous solution;
[0028] 2) Take 15g of hydroxyapatite ceramic particles with a particle size of 70-100 μm, add them to the above-mentioned sodium hyaluronate solution and stir evenly to form a paste compound (the solid content of the calcium phosphate ceramic particles is 60 (w / w)%, The solid content of sodium hyaluronate is 1.0 (w / w)%);
[0029] Preparation of hydroxyapatite ceramic particles with a particle size of 70-100 μm: disperse hydroxyapatite micropowder into 1.5% polyvinyl alcohol aqueous solution, transfer to a ball mill and mill for 24 hours to form a slurry with good particle dispersibility. The p...
Embodiment 2
[0031] Embodiment 2 Preparation of injectable calcium phosphate / collagen composite material of the present invention
[0032] 1. Preparation method
[0033] 1) Take medical collagen and prepare 10ml of 1.0% aqueous solution;
[0034] 2) Take 30g of biphasic calcium phosphate ceramic particles (hydroxyapatite / tricalcium phosphate=70 / 30) with a particle size of 40-70 μm, add them to the above collagen solution and stir evenly to form a paste-like composite (calcium phosphate ceramic The solid content of the particles is 75 (w / w)%, and the solid content of the collagen is 0.25 (w / w)%);
[0035] Preparation of biphasic calcium phosphate ceramic particles with a particle size of 40-70 μm: Disperse the biphasic calcium phosphate micropowder into a 1.0% polyvinyl alcohol aqueous solution, transfer to a ball mill and mill for 24 hours to form a slurry with good particle dispersion, through peristaltic The pump is transported to the spray dryer for spray drying treatment, the spray d...
Embodiment 3
[0037] Example 3 Preparation of injectable calcium phosphate / sodium hyaluronate-chondroitin sulfate composite material of the present invention
[0038] 1. Preparation method
[0039] 1) Take sodium hyaluronate (molecular weight 500kDa, Shandong Huaxi Freda Biomedicine Co., Ltd.) and chondroitin sulfate (Shanghai Aladdin Reagent Company) to prepare 10ml of 1.5% sodium hyaluronate and 0.5% chondroitin sulfate mixed aqueous solution;
[0040] 2) Take 30g of hydroxyapatite ceramic particles containing 5% bioactive glass (45S5 bioglass, Kunshan Huaqiao Technology New Material Co., Ltd.) with a particle size of 20-40 μm, and add it to the above-mentioned sodium hyaluronate-chondroitin sulfate mixture Stir evenly in the solution to form a paste compound (the solid content of calcium phosphate ceramic particles is 75 (w / w)%, and the solid content of sodium hyaluronate and chondroitin sulfate is 0.5 (w / w)%);
[0041] Preparation of hydroxyapatite ceramic particles containing 5% bioa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com