Inositol oxygenase mutant and application thereof
A technology of inositol oxygenase and mutants, applied in the field of bioengineering, can solve problems such as environmental pollution, use restrictions, and increase in glucaric acid production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
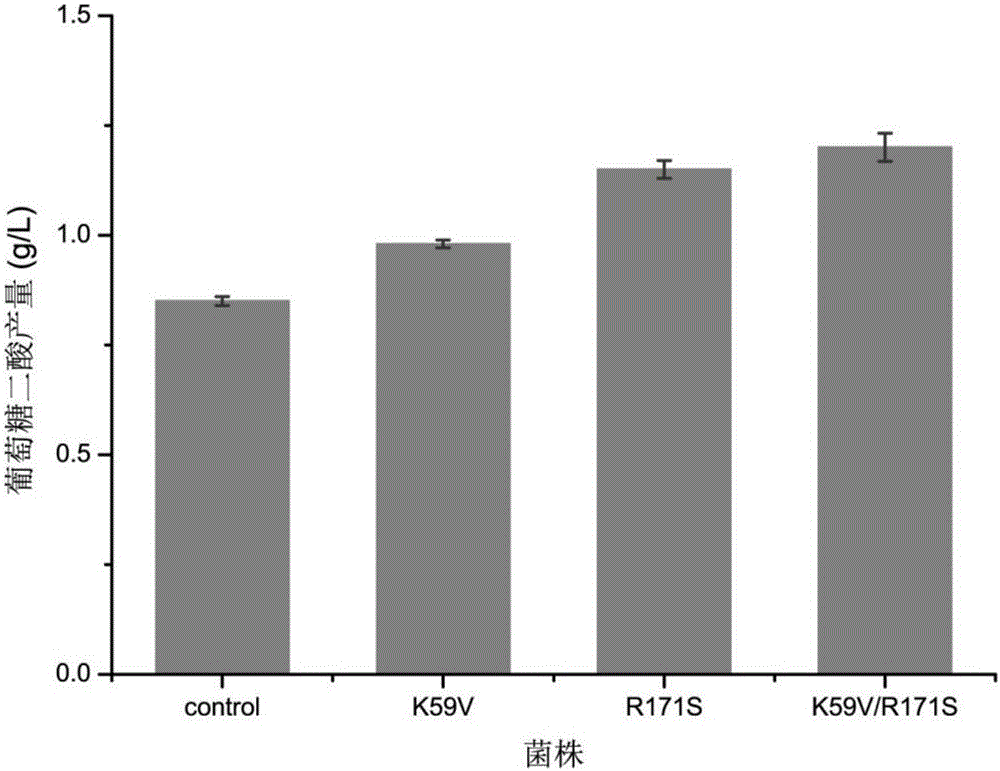
[0037] The construction of embodiment 1 inositol oxygenase miox mutant strain
[0038] Using site-directed mutagenesis kit (TakaRa), design two pairs of primers (as shown in Table 1), and use pUC57-miox (see Liu, Y., et al., Production of glucaric acid from myo-inositol inengineeredPichia pastoris.Enzyme for the plasmid construction method) and Microbial Technology, 2016.91: p.8-16.) as a template for PCR, the 59-position lysine inside the inositol oxygenase molecule is mutated to valine; or the 171-position arginine is mutated to serine , respectively named K59V and R171S. After the product was recovered, the transformation was verified, and the correct transformants were named pUC57-mioxK59V and pUC57-mioxR171S, respectively. Using the correctly sequenced strain pUC57-mioxK59V as a template, the arginine at position 171 inside the inositol oxygenase molecule was mutated to serine, and the correct transformant obtained was named pUC57-mioxK59V / R171S.
Embodiment 2
[0039] Example 2 Construction of recombinant Pichia pastoris pPIC9KGAP-Udh-mioxK59V, pPIC9KGAP-Udh-mioxR171S, pPIC9KGAP-Udh-mioxK59V / R171S
[0040] Using pGAPZB (commercialized plasmid, purchased by Invitrogen) as a template, using GAP-F\R as a primer pair to amplify the promoter, using the Pseudomonas putida genome as a template, using udh-F\R as a primer pair to amplify udh Gene (Gene ID: 1045739). At the same time, the pUC57-mioxK59V, pUC57-mioxR171S, and pUC57-mioxK59V / R171S plasmids were used as templates, and miox-F / R were used as primers to amplify the inositol oxygenase mutant gene respectively, and the fusion PCR method was used to combine the three mutant genes with The udh gene was fused, and restriction sites SacI and NotI were introduced into the upstream and downstream of the fusion fragment respectively. After double enzyme digestion and ligation transformation, after identification, the recombinant plasmid was linearized and electroporated into the expression ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com