Application of mogrol to preparation of antiviral drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
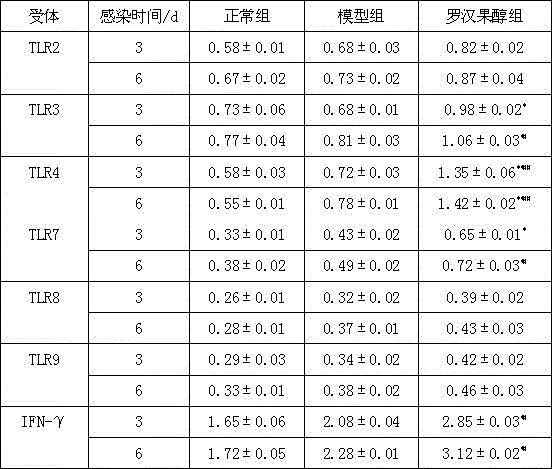
[0054] Example 1 Effect of Mogrosanol on the expression of TLRs receptors in suckling mice infected with rotavirus
[0055] 1. Experimental materials
[0056] LLC-MK2 cells (virus culture cells); simian rotavirus SA11 strain; natural delivery of suckling mice (meet clean-grade requirements, average body weight 1.88g, breastfeeding after birth, laboratory environment: SPF); 1640 medium; fetal cow Serum; trypsin; double antibody; rotavirus detection kit; Trizol total RNA extraction kit; SYBR Green qPCRSuperMix; cell growth medium (under strict sterile conditions, take 90ml of 1640 culture medium and add 10ml of fresh fetal bovine serum, 4 ℃ storage); virus maintenance solution (trypsin was added to 1640 medium, the concentration was 1 μg / ml); water-proof constant temperature incubator; ultra-low temperature refrigerator; optical microscope; electronic balance; liquid nitrogen tank; low-temperature centrifuge; fluorescent PCR instrument . All target gene sequences used were syn...
Embodiment 2
[0070] Example 2 Effect of Mogrosanol on Hepatitis C Virus Activity
[0071] 1. Experimental materials and instruments
[0072] Huh7.5.1 cells; plasmid pJFH1 containing the complete genome sequence of HCV2a type JFH1 virus strain; virus JFH1-5AGFP with reporter gene; DMEM medium (purchased from GIBCO); MTT detection reagent (purchased from biomol); Endonuclease (purchased from NEB); Zeiss advanced inverted microscope Axio observer A1 (Carl Zeiss); PerkinElmer multi-function detector; mogrosin (self-made, purity 98%).
[0073] Experimental method and results
[0074] 2.1 Construction of plasmid pJFH1-5AGFP and preparation of virus JFH1-5AGFP
[0075] Plasmid transformation was carried out on pJFH1, and a restriction site Xho (nt7523-nt7528, aa419-aa420) at the C-terminus of the NS5A coding region was selected and inserted into the EGFP gene (amplified from the PEGFP-N1 plasmid). After the plasmid was successfully constructed, it was sequenced and identified. Using the plasmi...
Embodiment 3
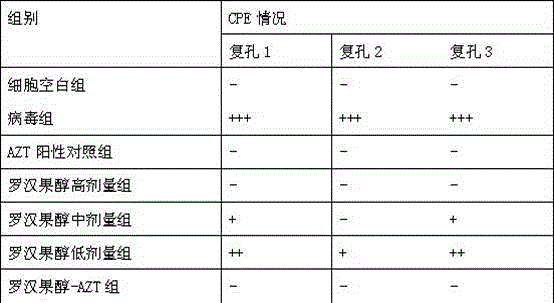
[0091] Example 3 Anti-HIV activity experiment of mogrosvenol
[0092] In this experiment, the cytopathic effect method (CPE) and RNA reverse transcription fluorescent quantitative PCR method were used to evaluate the anti-HIV effect of Mogrosanol in vitro.
[0093] 1 Materials and reagents
[0094]CEMxl74 cells and HIV-1 virus (both from the Aarond Diamond AIDS Research Center in the United States, donated by the Institute of Experimental Animals, Chinese Academy of Medical Sciences); DMSO (imported by the Academy of Military Medical Sciences); RPMI Medium 1640basic (1×), F etalBovine Serum (Shanghai Lifei Biotechnology Co., Ltd.); Zidovudine (AZT, 3'-Azido-3'-deoxythymidine) was purchased from Sigma. The mRNA quantitative PCR primers of each gene were synthesized by Shanghai Handsome Biotechnology Co., Ltd. Monk Fruit Alcohol (self-made, purity greater than 98%).
[0095] 2. Test method
[0096] 2.1 Cell culture
[0097] The CEMx174 cell line was inoculated in RPM11640 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com