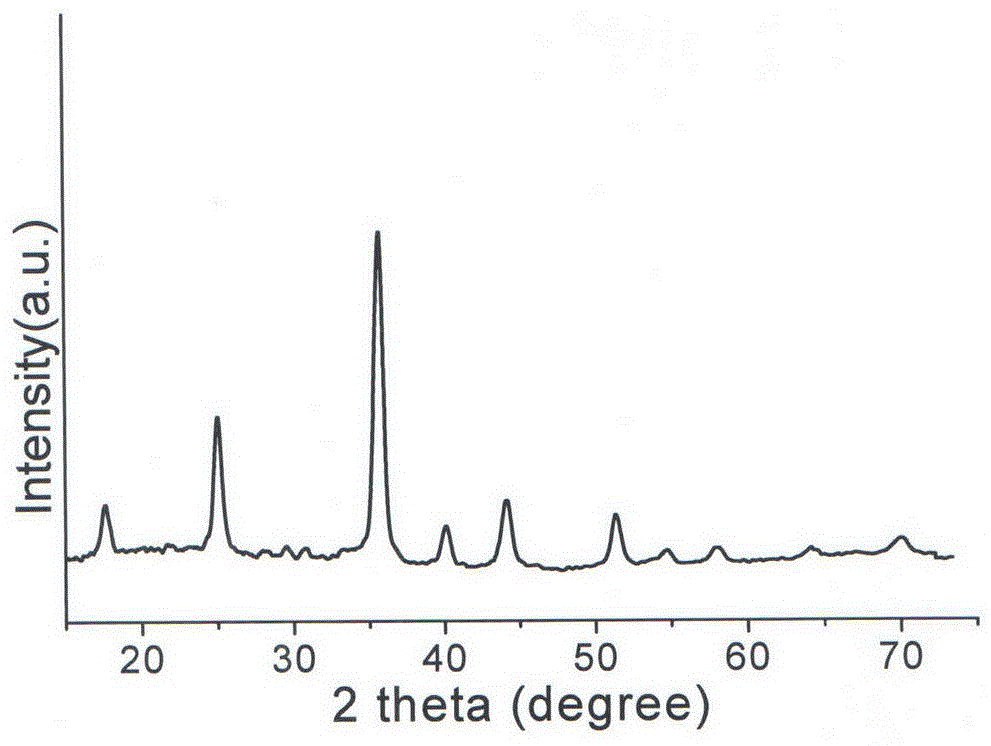
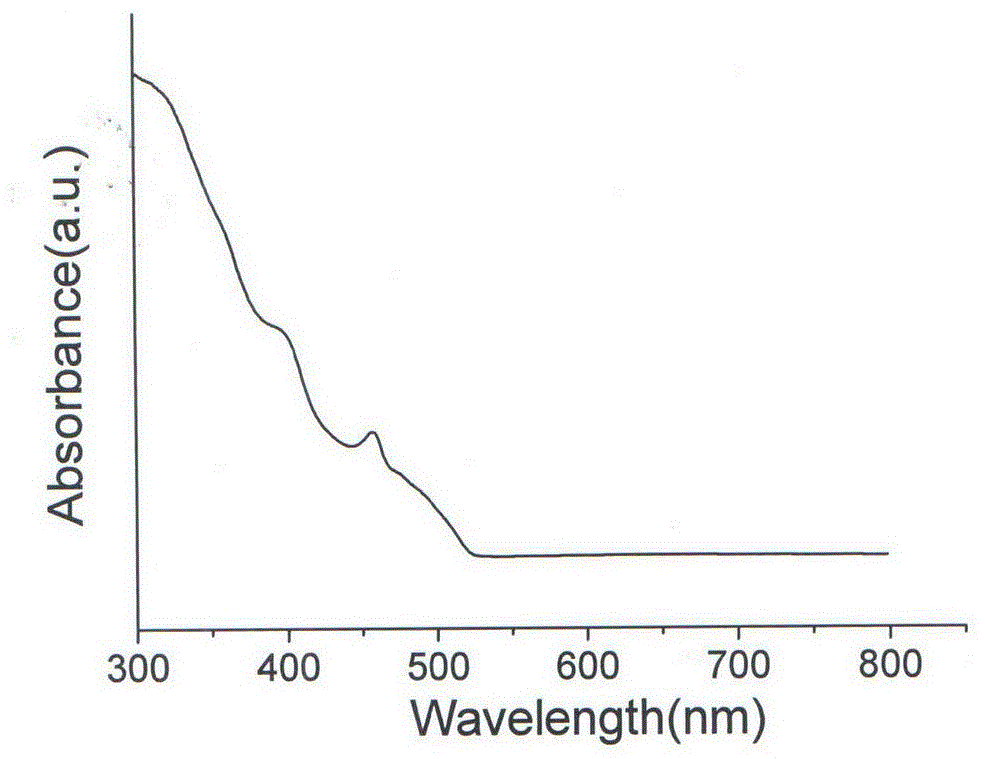
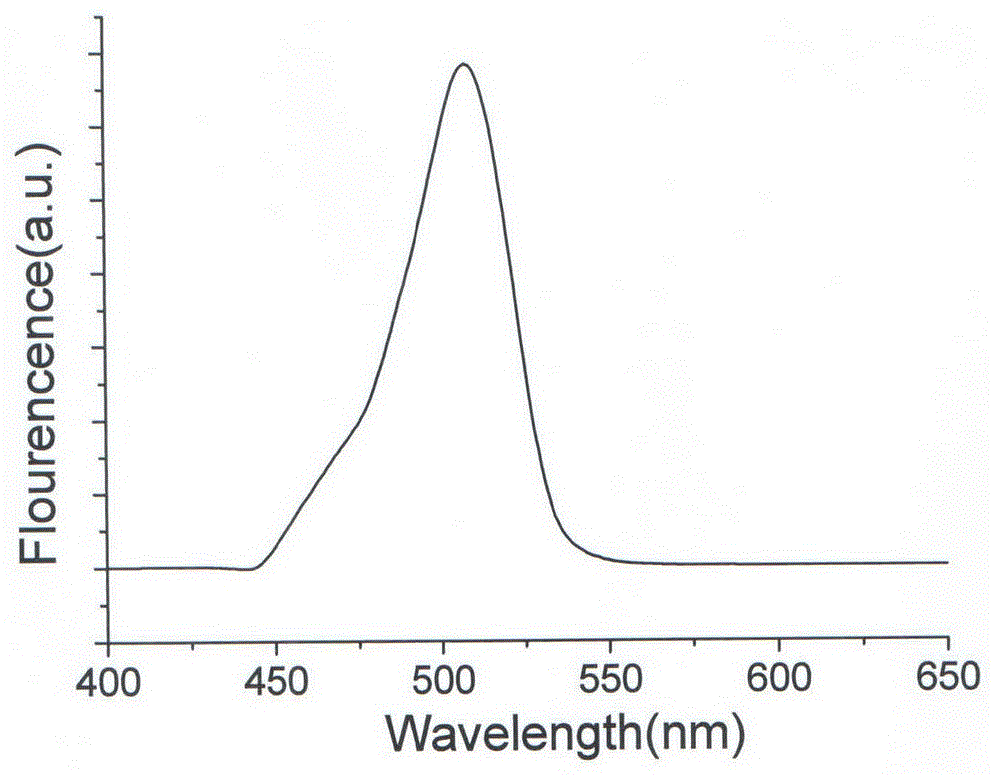
Copper-doped all-inorganic halogen perovskite fluorescent material and preparation method and application thereof
A technology of fluorescent materials and copper doping, which is applied in the fields of luminescent materials, chemical instruments and methods, nano optics, etc., and can solve problems such as not satisfying the B ion octahedral configuration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] a, 0.19mmoL of lead bromide (PbBr 2 ) and 0.01mmoL copper bromide (CuBr 2 ) powder in a 30mL quartz reactor, add 5mL of octadecene, then add 0.3mL of oleic acid and 0.3mL of oleylamine, seal with a rubber stopper, place in a magnetic stirring electric heating mantle, vacuumize, and slowly heat up to 100°C;
[0024] b. After constant temperature for 30 minutes, fill in nitrogen gas, slowly raise the temperature of the reaction system to 170°C, fill in nitrogen gas, and keep constant temperature for 30 minutes after the temperature stabilizes.
[0025] c. Rapidly inject 0.4 mL of octadecene solution containing 0.8 M cesium ions into the system of step b, react for 5 seconds, and quickly place the entire quartz reactor in an ice-water bath for 5 minutes;
[0026] d, the solution in step c was centrifuged at 9000 rpm in a high-speed centrifuge for 15 minutes,
[0027] e. Wash the centrifuged product in step d three times with toluene solvent, and dry it under vacuum at a...
Embodiment 2
[0030] a, 0.16mmoL of lead bromide (PbBr 2 ) and 0.04mmoL copper bromide (CuBr 2 ) powder in a 30mL quartz reactor, add 5mL of octadecene, then add 0.4mL of oleic acid and 0.4mL of oleylamine, seal with a rubber stopper, place in a magnetic stirring electric heating mantle, vacuumize, and slowly heat up to 105°C;
[0031] b. After constant temperature for 30 minutes, fill in nitrogen gas, slowly raise the temperature of the reaction system to 160°C, fill in nitrogen gas, and continue to keep constant temperature for 30 minutes after the temperature is stable;
[0032] c. Quickly inject 0.3mL of octadecene solution containing 0.1M cesium ions into the system of step b, react for 7 seconds, and quickly place the entire quartz reactor in an ice-water bath for 6 minutes,
[0033] d. Centrifuge the solution in step c at 8000 rpm for 15 minutes in a high-speed centrifuge;
[0034]e. Wash the centrifuged product in step d 4 times with n-hexane solvent, and dry it under vacuum at a...
Embodiment 3
[0037] a, 0.12mmoL of lead bromide (PbBr 2 ) and 0.08mmoL copper bromide (CuBr 2 ) powder in a 30mL quartz reactor, add 5mL of octadecene, then add 0.5mL of oleic acid and 0.5mL of oleylamine, seal with a rubber stopper, place in a magnetic stirring electric heating mantle, vacuumize, and slowly heat up to 110°C;
[0038] b. After constant temperature for 30 minutes, fill in nitrogen gas, slowly raise the temperature of the reaction system to 170°C, fill in nitrogen gas, and continue to keep constant temperature for 30 minutes after the temperature is stable;
[0039] c. Quickly inject 0.4mL of octadecene solution containing 0.5M cesium ions into the system of step b, react for 8 seconds, and quickly place the entire quartz reactor in an ice-water bath for 10 minutes;
[0040] d. Centrifuge the solution in step c at 10,000 rpm for 15 minutes in a high-speed centrifuge;
[0041] e. Wash the centrifuged product in step d 4 times with n-hexane solvent, and dry it under vacuum ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com