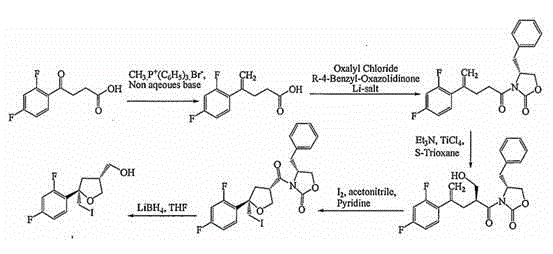
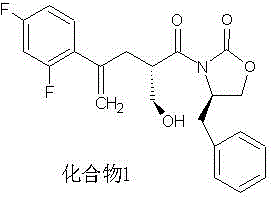
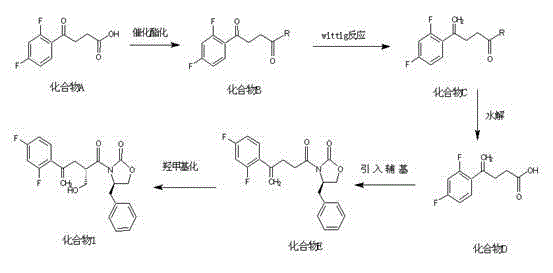
Method for preparing posaconazole intermediate
A technology for posaconazole and intermediates, which is applied in the preparation of organic compounds, carboxylate esters, and oxygen-containing compounds, and can solve problems such as poor solubility of salts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Preparation of Compound B
[0027] Compound A (M=214) 200g
[0028] Ethanol 400ml 2 times
[0029] Thionyl chloride (M=119ρ=1.638) 68ml 1 times
[0030] Install a magnetic stirrer and a thermometer into a 1000ml three-neck bottle, add posaconazole d-1200g, ethanol 400ml, stir and mix, and slowly add 68ml of thionyl chloride dropwise at room temperature. After the dropwise addition, the heating mantle was heated to 60-70° C. for reflux reaction for 2 hours, and the end point of the reaction was monitored by a thin-layer plate. (TLC: chloroform: methanol = 30: 1). After the reaction was completed, the reaction solution was evaporated to dryness under reduced pressure, and after evaporation, 150ml of toluene and 150ml of water were added and stirred to separate layers. Separate the organic and aqueous layers. The organic layer was washed with water (200ml×3), the aqueous layers were combined, extracted once with 100ml toluene, and the organic layers were combined....
Embodiment 2
[0059] (1) Preparation of Compound B
[0060] Compound A (M=214) 200g
[0061] Methanol 400ml 2 times
[0062] Thionyl chloride (M=119ρ=1.638) 70ml 1 times
[0063] Install a magnetic stirrer and a thermometer into a 1000ml three-necked bottle, add posaconazole d-1200g, methanol 400ml, stir and mix, and slowly add 70ml of thionyl chloride dropwise at room temperature. After the dropwise addition, the heating mantle was heated to 60-70° C. for reflux reaction for 2 hours, and the end point of the reaction was monitored by a thin-layer plate. (TLC: chloroform: methanol = 30: 1). After the reaction was completed, the reaction solution was evaporated to dryness under reduced pressure, and after evaporation, 150ml of toluene and 150ml of water were added and stirred to separate layers. Separate the organic and aqueous layers. The organic layer was washed with water (200ml×3), the aqueous layers were combined, extracted once with 100ml toluene, and the organic layers were combi...
example
[0075]
[0076] The preparation methods refer to Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com