The method and its application of extracting dibutyl phthalate from triangular
A technology of dibutyl phthalate and ethyl acetate, applied in the application of anti-lung cancer drugs, and the field of preparation of anti-lung cancer, can solve the problems of little research on small molecule pharmacodynamics, little research on small molecular compounds, etc., and achieve practical High performance, stable properties, and low reagent consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The present embodiment extracts the method for dibutyl phthalate monomer compound from triangular, comprises the following steps:
[0054] (1) Take the dried triangular ribs as the raw material, put them into a superfine pulverizer for superfine pulverization, and use 4°C water as the internal cooling liquid of the superfine pulverizer during the pulverization process, and the superfine pulverization time is 12 minutes to obtain superfine powder.
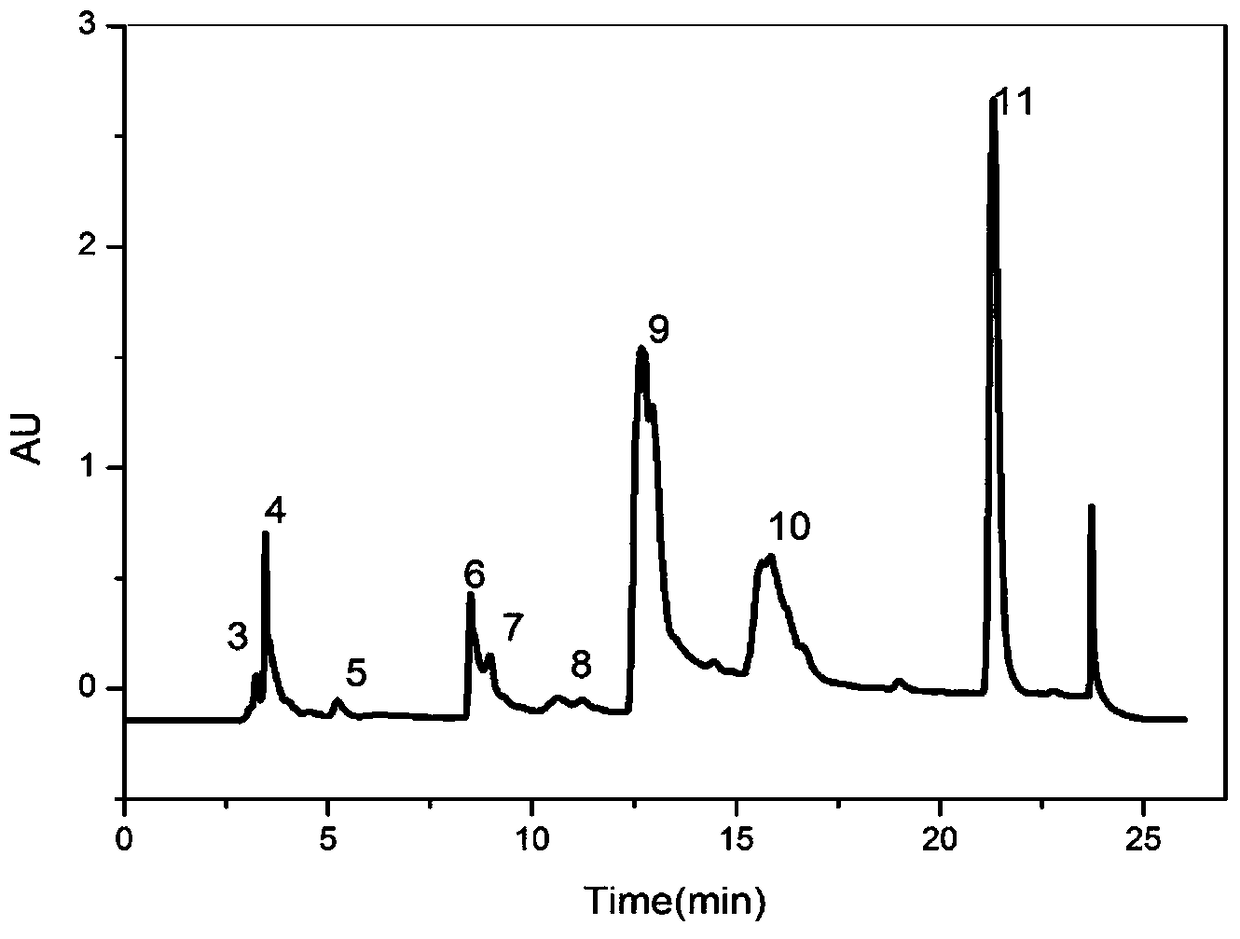
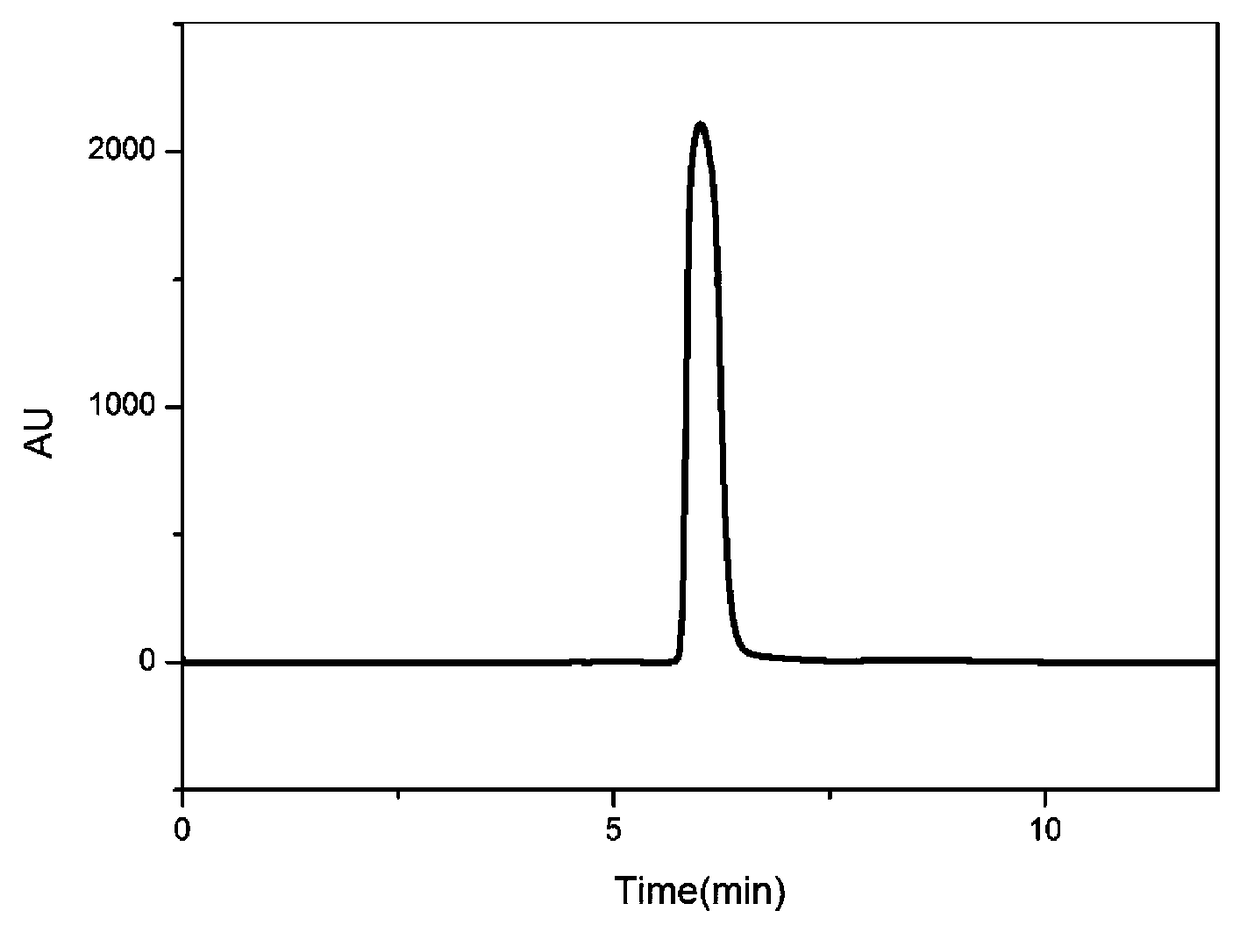
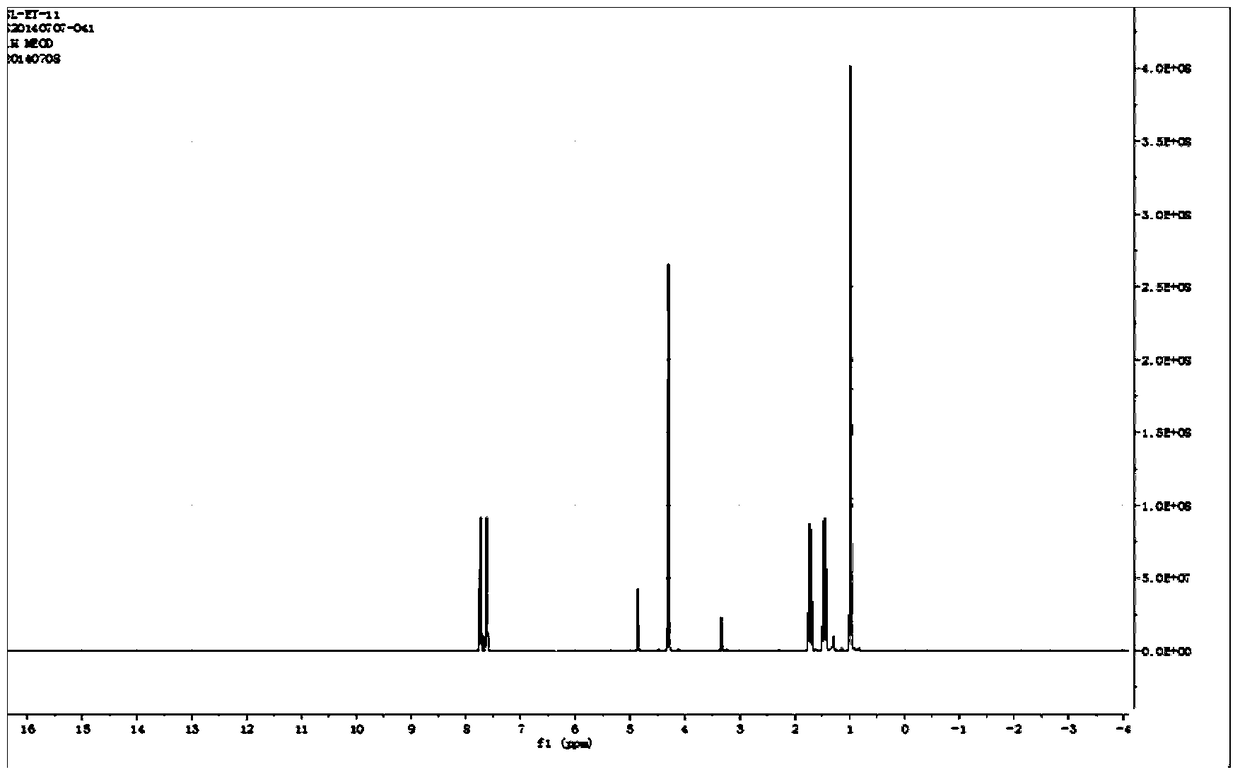
[0055] (2) Take 3Kg of the above-mentioned triangular superfine powder and extract it three times with ethyl acetate. For the first extraction: add 3kg of superfine powder to 24L ethyl acetate, extract for 70min, and after the extraction is completed, centrifuge at 25°C and 6000rpm for 30min to collect the residue. The second extraction: add the residue of the first extraction into 12L ethyl acetate, extract for 70min, and after the extraction is completed, centrifuge at 25°C and 6000rpm for 30min to collect the residue. The ...
Embodiment 2
[0062] Embodiment 2: the test of inhibiting the growth of human lung cancer A549 cells
[0063] Cell culture:
[0064] at 5% C0 2 , 37° C., under saturated humidity, cultured human lung cancer A549 cells with F-12K (10% FBS+1% PS) medium, and selected the cells growing in the logarithmic phase as experimental cells. After counting the cells, dilute it with medium to obtain a certain concentration of cell suspension.
[0065] Cell growth monitoring:
[0066] Put the cell real-time monitor in 5% C0 2 , 37°C saturated humidity incubator. Take an 8-well plate, add 150 μL of F-12K medium to each well, and put it into the real-time cell monitor for baseline. After the baseline, take out the eight-well plate and add 345 μL of diluted A549 cell suspension to each well to reach the number of cells in each well. About 2×10 4 Let stand for 3 minutes, and observe whether the cells are uniform under an inverted microscope. Add 5 μL of the diluted drug to each well to a final concent...
Embodiment 3
[0071] Embodiment 3: the test of suppressing the growth of human liver cancer Hep-G2 cells
[0072] Cell culture:
[0073] at 5% C0 2 , 37° C., under saturated humidity, cultured human lung cancer Hep-G2 cells with MEM (10% FBS, 1% PS) medium, and selected cells with good growth status as experimental cells. After counting the cells, dilute it with medium to obtain a certain concentration of cell suspension.
[0074] Cell growth monitoring:
[0075] Put the cell real-time monitor in 5% C0 2 , 37°C saturated humidity incubator. Take an 8-well plate, add 150 μL of MEM medium to each well, put it into a real-time cell monitor for baseline, take out the eight-well plate after the baseline, and add 345 μL of diluted Hep-G2 cell suspension to each well to reach the number of cells in each well. About 4×10 4 Let stand for 3 minutes, and observe whether the cells are uniform under an inverted microscope. Add 5 μL of the diluted drug to each well until the final concentration of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com