Anti-infective drug cefetamet pivoxil hydrochloride composite capsule
A technology of ceftazime pivoxil hydrochloride and a composition, which is applied in the field of anti-infective drug ceftazime pivoxil hydrochloride composition capsules, can solve the problems of patient harm, immediate allergic reaction, etc., achieves low polymer content, significant antibacterial Active and low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
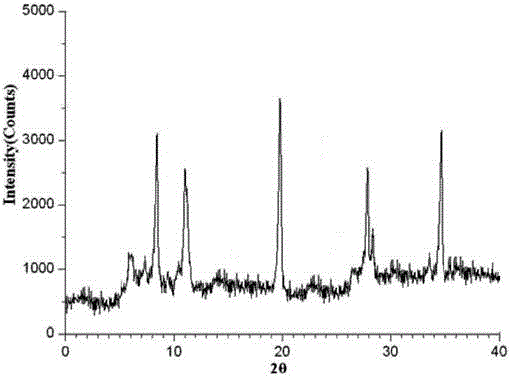
[0033] Example 1: Preparation of ceftazidime pivoxil hydrochloride crystal
[0034] 1) Prepare mixed solution A by mixing dimethylformamide and water at a volume ratio of 4:1;
[0035] 2) Take ceftazidime pivoxil hydrochloride raw material, add the mixed solution A prepared in step 1), wherein the ratio of the volume of the mixed solution A to the mass of ceftazime pivoxil hydrochloride is 8ml:1g, stir to dissolve all Add 0.1% g / ml activated carbon to the resulting solution for decolorization and filtration to obtain a clear solution;
[0036] 3) Prepare mixed solution B with isopropyl ether and isopropanol at a volume ratio of 3.5:1;
[0037] 4) At room temperature, add mixed solution B to the clear solution obtained in step 2) under an ultrasonic field with a power of 0.6KW, where the amount of mixed solution B added is 7 times the volume of mixed solution A, and turn off the ultrasonic field after adding , cooled to 3° C., allowed to stand for 2 hours, crystals were pre...
Embodiment 2
[0039] Example 2: The preparation of ceftazime pivoxil hydrochloride capsules, the steps are as follows:
[0040] Prescription: in parts by weight as shown in Table 1
[0041] Table 1 Ceftazidime hydrochloride composition prescription
[0042]
[0043] Preparation:
[0044] 1) Processing of raw and auxiliary materials: use a vibrating sieving machine to pass microcrystalline cellulose and magnesium aluminum silicate through a 60-mesh sieve, and ceftazidime pivoxil hydrochloride through a 80-mesh sieve;
[0045] 2) Weighing: Weighing according to the prescription;
[0046] 3) Adhesive preparation: Add povidone K30 into absolute ethanol, stir to dissolve evenly, and set aside;
[0047] 4) Granulation: Add ceftazidime pivoxil hydrochloride, microcrystalline cellulose, and magnesium aluminum silicate to the wet granulator, turn on the stirring motor and dry mix for 5 minutes, add povidone K30 ethanol solution, wet mix and cut 100- 150 seconds to make soft material, 18-mes...
Embodiment 3
[0052] Example 3: The preparation of ceftazime pivoxil hydrochloride capsules, the steps are as follows:
[0053] Prescription: in parts by weight as shown in Table 2
[0054] Table 2 Ceftazidime hydrochloride composition prescription
[0055]
[0056] Preparation:
[0057] 1) Processing of raw and auxiliary materials: use a vibrating sieving machine to pass microcrystalline cellulose and magnesium aluminum silicate through a 60-mesh sieve, and ceftazidime pivoxil hydrochloride through a 80-mesh sieve;
[0058] 2) Weighing: Weighing according to the prescription;
[0059] 3) Adhesive preparation: Add povidone K30 into absolute ethanol, stir to dissolve evenly, and set aside;
[0060] 4) Granulation: Add ceftazidime pivoxil hydrochloride, microcrystalline cellulose, and magnesium aluminum silicate to the wet granulator, turn on the stirring motor and dry mix for 5 minutes, add povidone K30 ethanol solution, wet mix and cut 100- 150 seconds to make soft material, 18-mes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com