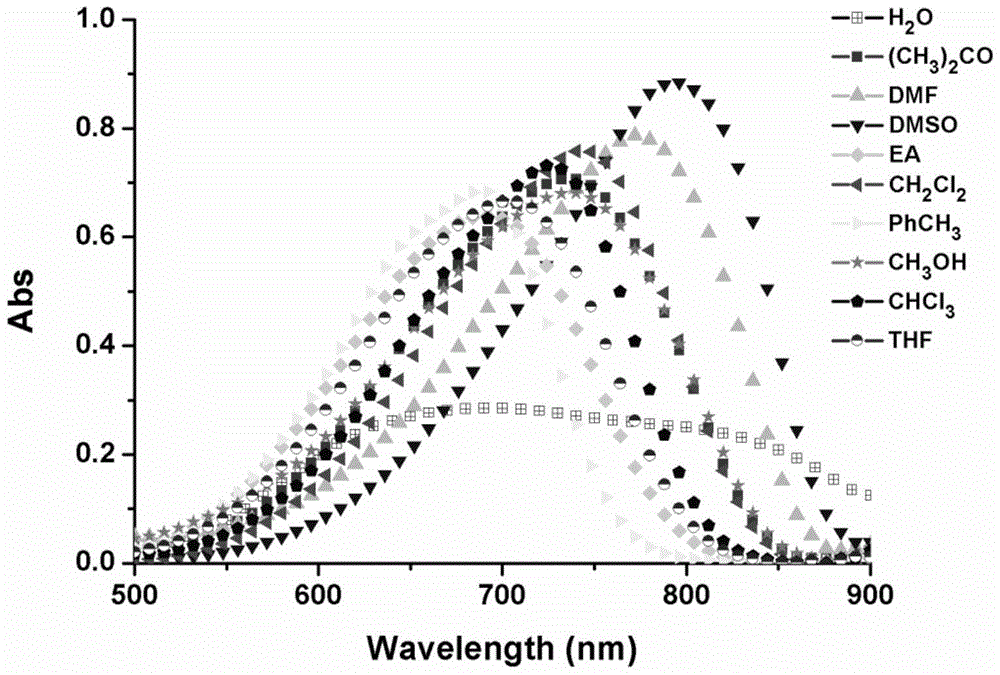
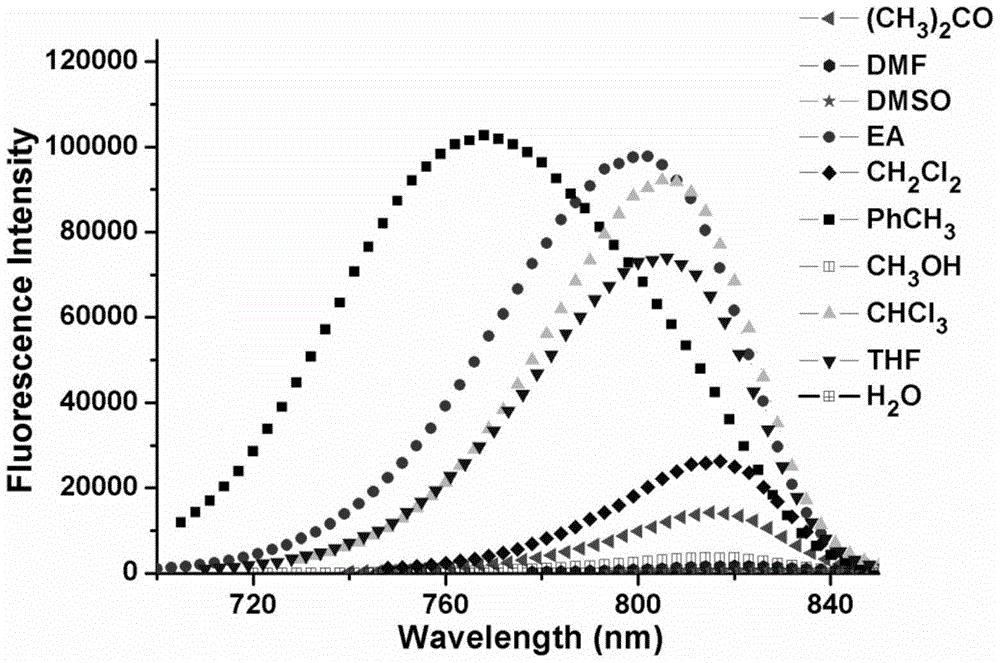
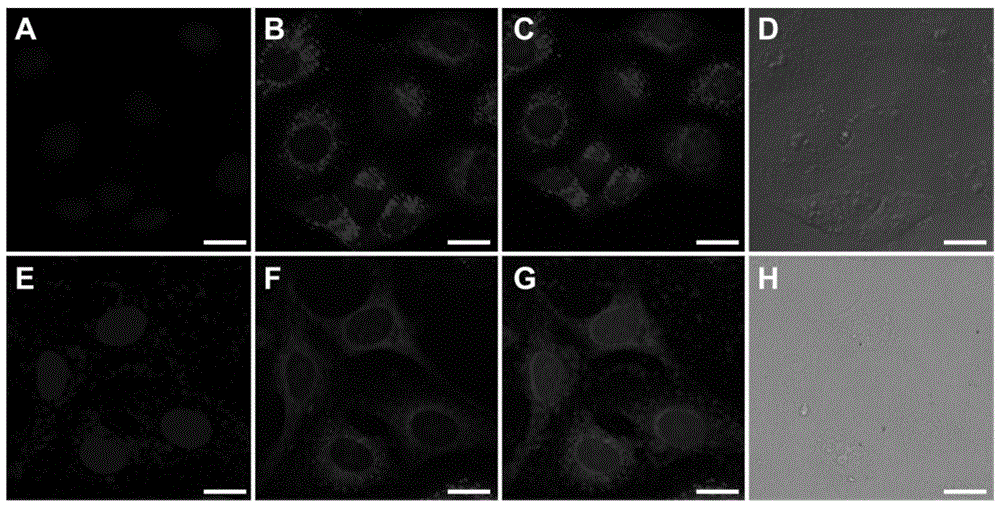
A kind of near-infrared fluorescent probe sensitive to environmental polarity and its synthesis method and application
A synthesis method and fluorescent probe technology, applied in the field of synthesis and medicine, to achieve the effects of improved accuracy, simple imaging operation process, and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of compound I:
[0037] Indole iodide salt (0.63g, 2mmol) and condensing agent (0.34g, 2mmol) were dissolved in toluene 30mL, acetic acid 10mL mixed solution, reacted at 110°C for 2h; after the reaction, concentrated under reduced pressure at 60°C for 30min to remove the solvent, separated by column , and the eluent was dichloromethane:methanol volume ratio 50:1 to obtain 0.42 g of a black-red solid, namely compound I (yield 60%).
[0038] Synthesis of compound II:
[0039] Benzoylacetone (0.48g, 3mmol) was dissolved in 15mL of diethyl ether, 1mL of boron trifluoride diethyl ether solution was dropped into the above mixed solution, refluxed and stirred at 40°C for 2h, after the reaction was completed, cooled to room temperature, a large amount of powder was precipitated, and suction filtered 0.54 g of light yellow solid powder was compound II (yield 85%).
[0040] Under the protection of argon, compound I (0.341g, 1mmol) and compound II (0.315g, 1.5mmol) wer...
Embodiment 2
[0042] Under the protection of argon, compound I (0.171g, 0.4mmol) and compound II (0.158g, 0.5mmol) were dissolved in acetic anhydride 5mL, and anhydrous sodium acetate (0.041g, 0.4mmol) was added to obtain a mixed solution, 55 ℃ reaction 1h. After completion of the reaction, cool to room temperature, add 15mL of 20% sodium bicarbonate solution into the above mixture, and precipitate black-green insoluble matter, re-dissolve the black-green insoluble matter with dichloromethane (8mL), and use column chromatography Separation (the eluent is a mixed solution of dichloromethane and methanol, the volume ratio of dichloromethane and methanol is 90:1), and 0.12 g (45% yield) of a golden-green solid is obtained, which is the near-infrared fluorescent probe MCY -BF.
Embodiment 3
[0044] Under the protection of argon, compound I (0.100g, 0.29mmol) and compound II (0.091g, 0.43mmol) were dissolved in acetic anhydride 10mL, and anhydrous sodium acetate (0.021g, 0.26mmol) was added to obtain a mixed solution, 80 ℃ reaction 3h. After the reaction was completed, cool to room temperature, add 10 mL of 30% sodium bicarbonate solution into the above mixture, and a black-green insoluble matter was precipitated, and the black-green insoluble matter was redissolved with dichloromethane (5 mL), and analyzed by column chromatography Separation (the eluent is a mixed solution of dichloromethane and methanol, the volume ratio of dichloromethane and methanol is 110:1), and 0.063g (40% yield) of a golden-green solid is obtained, which is the near-infrared fluorescent probe MCY -BF.
[0045] Characterization by melting point, infrared, NMR and mass spectrometry
[0046] Probe melting point (m.p.):250-253℃
[0047] Infrared absorption characteristic peak (IR): V C-H =...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com