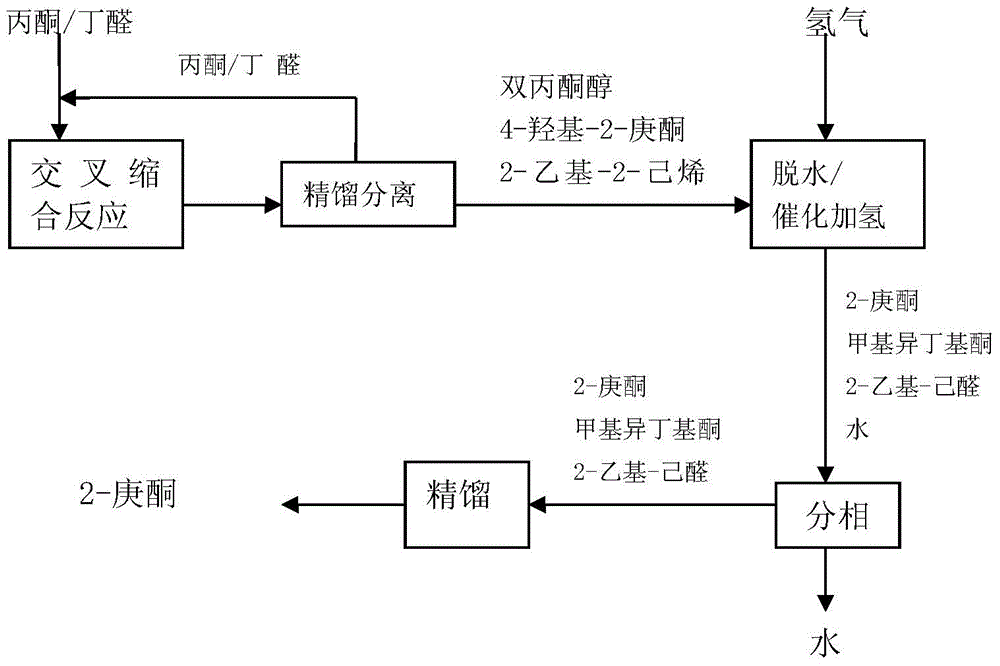
Synthetic method of 2-heptanone
A synthesis method and technology of acetone, applied in the field of synthesizing 2-heptanone, can solve the problems of many by-products, high production cost, and high equipment requirements, and achieve the effects of reducing the probability of side reactions, reducing equipment investment, and shortening the technological process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] According to attached figure 1 As shown in the process flow, in a single-tube reactor with a jacket (inner diameter of 10 mm), SiC pellets, 250 g of hydrogen-oxygen type macroporous strongly basic type I anion exchange resin and SiC pellets were respectively loaded, and the jacket Condensed water was added to control the reaction temperature. Acetone, butyraldehyde and water were fed into the reactor with a metering pump. The reaction products were analyzed by gas chromatography. Reaction conditions and results are shown in Table 1
[0033]
[0034]
[0035] Note: C6 is acetone self-condensation product and its partial dehydration product
[0036]C7 is the condensation product of acetone and butyraldehyde and its partial dehydration product
[0037] C8 is the self-condensation product of butyraldehyde and its partial dehydration product
Embodiment 2
[0039] The technical process identical with embodiment 1, in the there-necked flask that has stirrer, thermometer and dropping funnel, add 30ml massfraction and be 10% sodium hydroxide solution, add 350g acetone butyl mixed in a certain proportion through dropping funnel For the aldehyde mixture, control the dropwise addition time for about 2 hours. After the dropwise addition, continue to stir and react for 15 minutes, and then add a small amount of sulfuric acid to neutralize the reaction solution to make it neutral. The product was analyzed by gas chromatography, and the results are shown in Table 2.
[0040] Table 2
[0041]
Embodiment 3
[0043] The same technological process as embodiment 1, in the there-necked flask with stirrer, thermometer, dropping funnel, add 100ml concentration and be the sulfuric acid solution of 1mol / L, and adorn on the there-necked flask with the condensing tube of receiving bottle, condense Put condensed water into the tube, then add the three-necked flask to 100-120°C, then add 150g diacetone alcohol and 150g 4-hydroxy 2-heptanone dropwise through the dropping funnel within about 3 hours, and receive the dehydration product isopropyl through the condenser tube Acetone, 3-hepten-2-one and the water taken out by azeotropy continued to react for about 5 hours after the dropwise addition was completed. During the reaction, the water phase collected in the receiving bottle was intermittently added back to the three-necked flask to continue the reaction, and the oil phase was the products mesityl oxide and 3-hepten-2-one. After the reaction, the aqueous phase was extracted with ether, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com