Immunologic adjuvant and vaccine containing immunologic adjuvant
An immune adjuvant and immune vaccine technology, which is applied in the field of immune adjuvants and vaccines containing the immune adjuvant, can solve the problems of flocculation, poor adjuvant stability, unfavorable production and practical application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
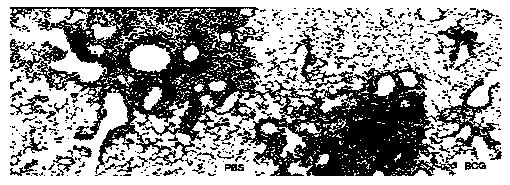
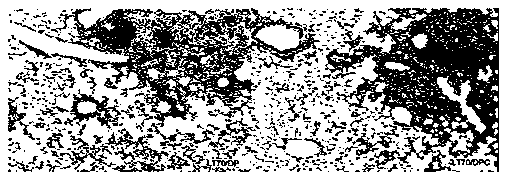
[0081] Preparation and performance verification of immune adjuvant and immune vaccine of the present invention
[0082] 1. Adjuvant DDA and PolyI:C adjuvant screening
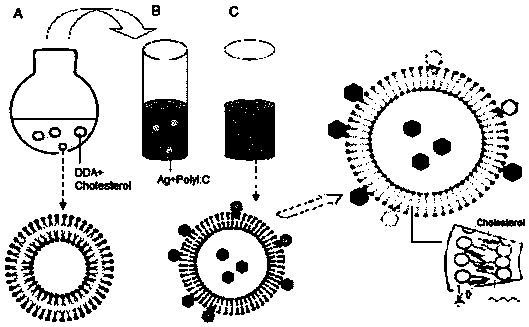
[0083] DDA is a cationic liposome, which self-assembles into a liposome when the temperature is heated to a gel liquid higher than its phase transition temperature, and the liposome in the complex cationic gel state and the oppositely charged polymyocyte PolyI:C usually Lead to high instability, easy to form flocculation phenomenon. This patent improves the stability of the adjuvant DDA and PolyI:C by adding different auxiliary materials to stabilize the adjuvant DDA and PolyI:C.
[0084]In the selection of different excipients, we selected excipients such as Tween 80, Span 85, gelatin, cholesterol, lecithin, β-cyclodextrin, PLGA and other excipients, and tested their effect on the particle size and Zeta potential. Improved effect of adjuvants DDA and PolyI:C. The liposomes formed by adding different adjuvan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com