Specific fluorescent probe for identifying hydrogen sulfide and application of probe
A fluorescent probe, hydrogen sulfide technology, applied in fluorescence/phosphorescence, luminescent materials, organic chemistry, etc., can solve problems such as damage to the sample to be tested, inability to achieve sulfide detection, achieve good fluorescence emission spectral characteristics, and simple synthesis process Easy, highly sensitive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
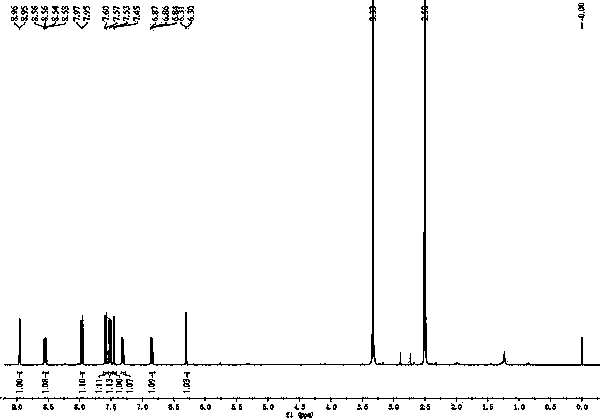
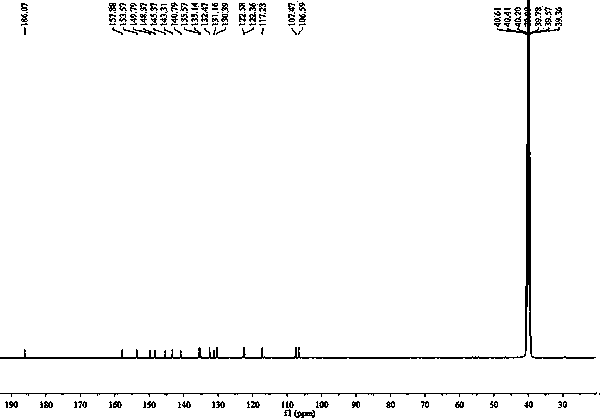
[0027] Example 1 The chemical synthesis of 7-(2,4-dinitrophenoxy)-3H-phenoxazin-3-one
[0028] Figure 10 Shown in 7-(2,4-dinitrophenoxy)-3H-phenoxazin-3-one synthetic route, its synthetic steps are as follows:
[0029] (1) Dissolve 0.5 mmol of resorufin sodium salt, 0.625 mmol of potassium carbonate, and 0.5 mmol of 2,4-dinitrobromobenzene in 10 mL of N,N-dimethylformamide, and react at 80 °C for 4 h;
[0030] (2) Pour the reaction solution into ice water, a large amount of solid precipitates, and filter;
[0031] (3) The solid was purified by silica gel chromatography, and eluted with ethyl acetate-n-hexane (1: 1 v / v), to obtain 159.6 mg of orange solid powder;
Embodiment 2
[0032] Example 2 The selectivity of 7-(2,4-dinitrophenoxy)-3H-phenoxazin-3-one for different substances
[0033] (1) Prepare 99 μl metabolic reaction system in advance, including PBS buffer (10 mM) at pH 7.4: ethanol (volume ratio 9:1), fluoride ion (100 μM), chloride ion (50 μM), bromide ion (100 μM), L-iodide ion (100μM), sodium ion (100μM), potassium ion (100μM), calcium ion (100μM), magnesium ion (100μM), nitrate ion (100μM), sodium hydrosulfide (100μM)
[0034] (2) Add 1 μl of 7-(2,4-dinitrophenoxy)-3H-phenoxazin-3-one at a final concentration of 10 μM to the reaction system to initiate the reaction;
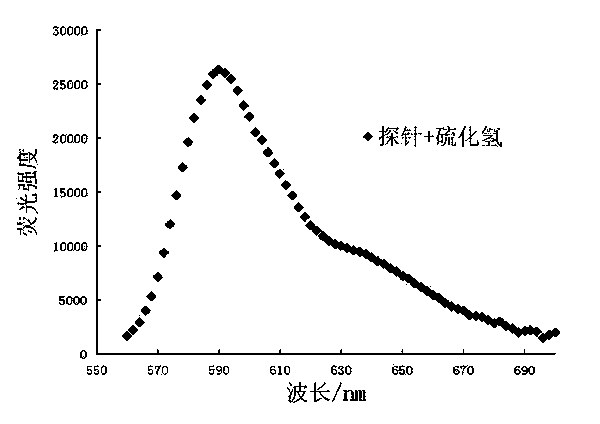
[0035] (3) After 60 min, perform fluorescence detection (λ Ex =550 nm, λ Em =590 nm); calculate the fluorescence intensity in each system (see Image 6 );
Embodiment 3
[0036] Example 3 Linear relationship between 7-(2,4-dinitrophenoxy)-3H-phenoxazin-3-one and hydrogen sulfide concentration
[0037] (1) Prepare 99 μl metabolic reaction system in advance, including PBS buffer (10 mM) at pH 7.4: ethanol (volume ratio 9:1), sodium hydrosulfide (0-200 μM), react at 37°C for 30 minutes ;
[0038] (2) Add 1 μl of 7-(2,4-dinitrophenoxy)-3H-phenoxazin-3-one at a final concentration of 10 μM to the reaction system to initiate the reaction;
[0039] (3) After 60 min, perform fluorescence detection (λ Ex =550 nm, λ Em =590 nm); calculate the fluorescence intensity in each system, establish the standard curve of fluorescence intensity and hydrogen sulfide concentration, the standard curve is y = 243.72x - 2422.7, where, y represents the fluorescence intensity, x represents the concentration of hydrogen sulfide (see Figure 7 );
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com