A kind of method for preparing drug sustained-release material
A technology for drug preparation and sustained release, which is applied in the field of biodegradable polymer drug sustained release material preparation, can solve the problems of difficult to flexibly and effectively control the dosing scheme, and difficult to meet the release requirements of drugs with different solubility properties, and achieves broad industrialization and market. Prospects, product quality indicators are stable, and the effect of improving mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] A polycaprolactone (matrix) / polyethylene oxide (disperse phase) drug sustained-release composite material comprises the following components and contents in parts by weight:
[0061]
[0062] Explanation: the weight of cephalexin (being a drug) is 1% of the total weight of the biodegradable polymer matrix and the dispersed phase.
[0063] The first step, at first prepare raw materials by above-mentioned components;
[0064] In the second step, the drug is dried in a vacuum oven at 60°C for 6 hours;
[0065]In the third step, the dried cephalexin, polycaprolactone and polyethylene oxide obtained in the second step are placed in a high mixer and premixed for 5 minutes at a speed of 100 rpm to obtain a drug and polymer premix , dry the premixed composite particles in a vacuum oven at 50°C for 3 hours.
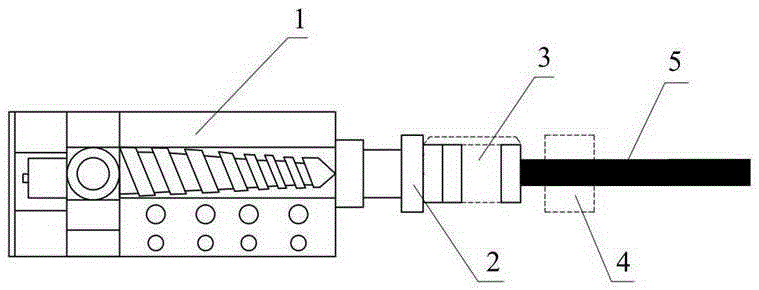
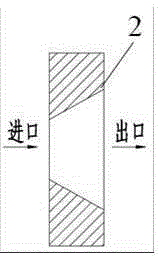
[0066] In the fourth step, the drug and the polymer premix obtained in the third step are put into figure 1 The single-screw extruder 1 of the integrated biaxially st...
Embodiment 2
[0071] A polylactic acid (matrix) / polyethylene oxide (dispersed phase) drug sustained-release composite material comprises the following components and their contents in parts by weight:
[0072]
[0073] Explanation: the weight of diclofenac sodium (being a medicine) is 5% of the total weight of the biodegradable polymer matrix and the dispersed phase.
[0074] The first step, at first prepare raw materials by above-mentioned components;
[0075] In the second step, the drug is dried in a vacuum oven at 60°C for 6 hours;
[0076] In the third step, the dried diclofenac sodium, polylactic acid and polyethylene oxide obtained in the second step are premixed together in a high-speed mixer for 5 minutes at a speed of 100 rpm to obtain a drug and polymer premix. Then put the obtained drug and polymer premix into a twin-screw extruder for melt blending, extrusion, and granulation to obtain premixed composite granules of polylactic acid / polyethylene oxide / diclofenac sodium, and ...
Embodiment 3
[0081] A polycaprolactone-polylactic acid (matrix) / polyethylene oxide (disperse phase) drug sustained-release composite material comprises the following components and contents by weight:
[0082]
[0083] Explanation: the weight of ibuprofen cephalexin (being medicine) is 20% of the total weight of the biodegradable polymer matrix 1-2 and the dispersed phase.
[0084] The first step, at first prepare raw materials by above-mentioned components;
[0085] In the second step, the drug is dried in a vacuum oven at 60°C for 6 hours;
[0086] In the third step, the dried ibuprofen, polycaprolactone, and polylactic acid obtained in the second step are put into a twin-screw extruder for melt blending, extrusion, and granulation to obtain premixed granules of medicine and polymer. The mixed composite particles were dried in a vacuum oven at 50°C for 3 hours, and the temperatures of the feeding port, conveying section, melting section, homogenizing section, and die of the twin-screw ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com