Derivative of aza-aryl compound
A nitrogen-heteroaryl compound technology, applied in the field of nitrogen-containing heteroaryl compound derivatives, can solve the problems of affecting drug efficacy and low solubility, and achieve the effects of increased solubility, good stability, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
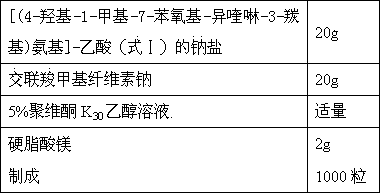
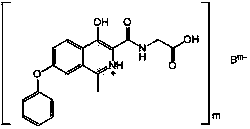
[0056] Example 1 Preparation of hydrochloric acid addition salt of [(4-hydroxyl-1-methyl-7-phenoxy-isoquinoline-3-carbonyl)amino]-acetic acid (formula Ⅰ)
[0057] 1 g of [(4-hydroxy-1-methyl-7-phenoxy-isoquinoline-3-carbonyl)amino]-acetic acid was dissolved in 10 mL of dichloromethane and stirred at room temperature. 0.11 g of hydrochloric acid was added dropwise to the reaction solution. After stirring the reaction solution at room temperature for 1 h, the reaction solution was evaporated to dryness, washed with n-hexane and dried in vacuo. Thus, 0.98 g of white solid was obtained, yield 89.3%. ESI-MS (M+H, 354.5), elemental analysis: C 58.69%, H 4.41%, N 7.21%, O2 0.58%, Cl 9.12%.
[0058] 1 H-NMR (DMSO) δ: 3.14 (s, 3H), 3.60 (d, 2H), 5.35 (s, -OH), 7.25 (s, 1H), 7.29 (s, 1H), 7.14 (s, 2H) , 7.41 (s, 2H), 7.17 (s, 1H), 8.03 (t, 1H), 8.09 (s, 1H).
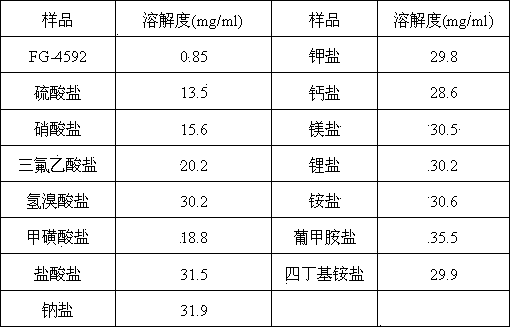
[0059] The preparation method of the acid addition salt of FG-4592 combined with sulfuric acid, benzenesulfonic acid, nit...
Embodiment 2
[0062] Example 2 Preparation of [(4-hydroxyl-1-methyl-7-phenoxy-isoquinoline-3-carbonyl)amino]-acetic acid (formula Ⅰ) sodium salt
[0063] Dissolve 1 g of [(4-hydroxy-1-methyl-7-phenoxy-isoquinoline-3-carbonyl) amino]-acetic acid in 10 mL of dichloromethane, add 1.1 equivalents of 10% aqueous sodium hydroxide to in the reaction solution. After stirring the reaction solution at room temperature for 1 h, the dichloromethane reaction solution was evaporated to dryness, washed with n-hexane and dried in vacuo. Obtained 0.99 g of white solid, yield 92.8%. ESI-MS (M-H, 352.5), elemental analysis: C 60.96%, H 4.04%, N 7.48%, Na 6.14%, O 21.37%.
[0064] 1 H-NMR (DMSO) δ: 3.14 (s, 3H), 4.21 (d, 2H), 5.35 (s, -OH), 7.25 (s, 1H), 7.29 (s, 1H), 7.14 (s, 2H) , 7.41 (s, 2H), 7.17 (s, 1H), 8.03 (t, 1H), 8.09 (s, 1H).
[0065] [(4-Hydroxy-1-methyl-7-phenoxy-isoquinoline-3-carbonyl)amino]-acetic acid and magnesium hydroxide, calcium hydroxide, lithium hydroxide, potassium hydroxide to...
Embodiment 3
[0066] Example 3 Preparation of [(4-hydroxyl-1-methyl-7-phenoxy-isoquinoline-3-carbonyl)amino]-ammonium salt of acetic acid (formula Ⅰ)
[0067] Add 1 g of [(4-hydroxy-1-methyl-7-phenoxy-isoquinoline-3-carbonyl)amino]-acetic acid into dichloromethane and stir at room temperature, fill with ammonia gas, react for 3 hours, and prepare a thin layer Detection, after the reaction was completed, evaporated to dryness to obtain 0.948 g of white solid, yield 90.5%. ESI-MS (M-H, 352.5); elemental analysis: C 61.78%, H 5.18%, N 11.38%, O21.66%;
[0068] 1 H-NMR (DMSO) δ: 2.86 (s, 3H), 3.14 (s, 3H), 3.37-4.0 (t, 4H), 3.56 (d, 1H), 3.58 (s, -OH), 3.85 (d, 2H), 5.35 (s, -OH), 7.25 (s, 1H), 7.29 (s, 1H), 7.14 (s, 2H), 7.41 (s, 2H), 7.0 (d, 2H), 7.17 (s, 1H), 7.16 (s, 4H), 8.03 (t, 1H), 8.09 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com