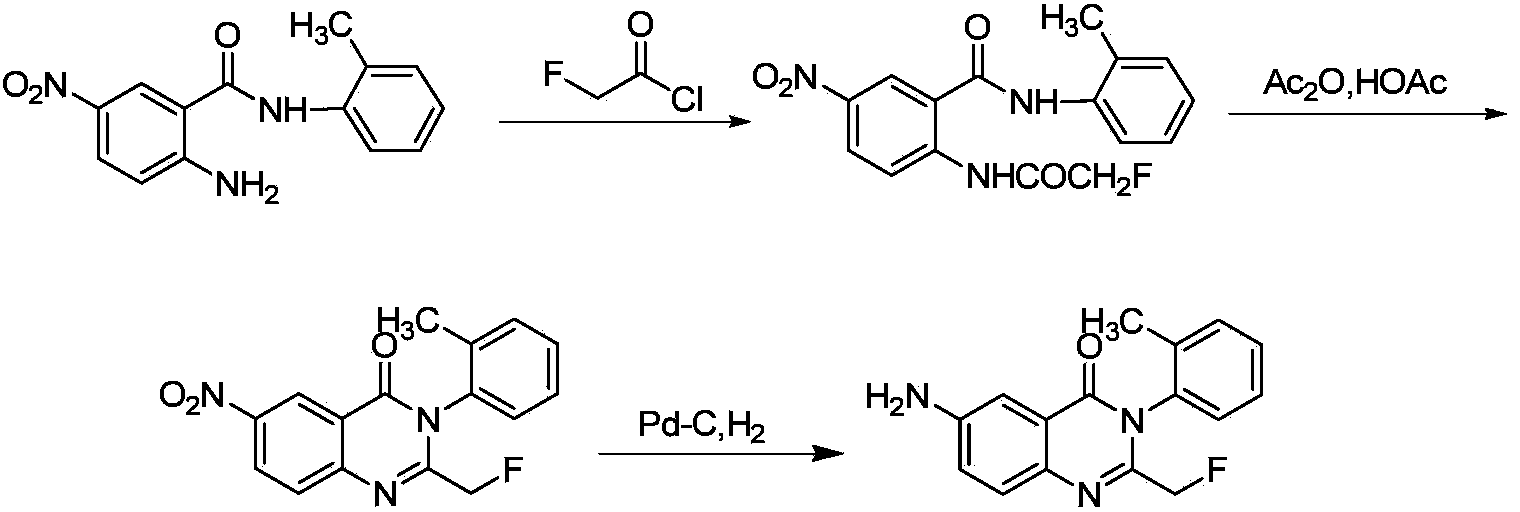
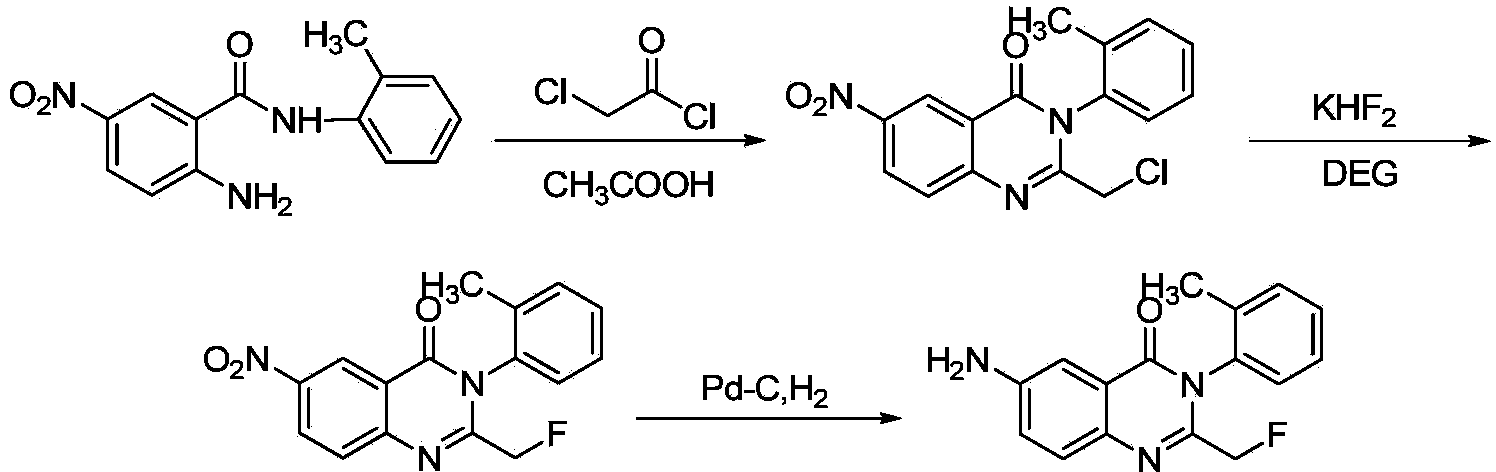
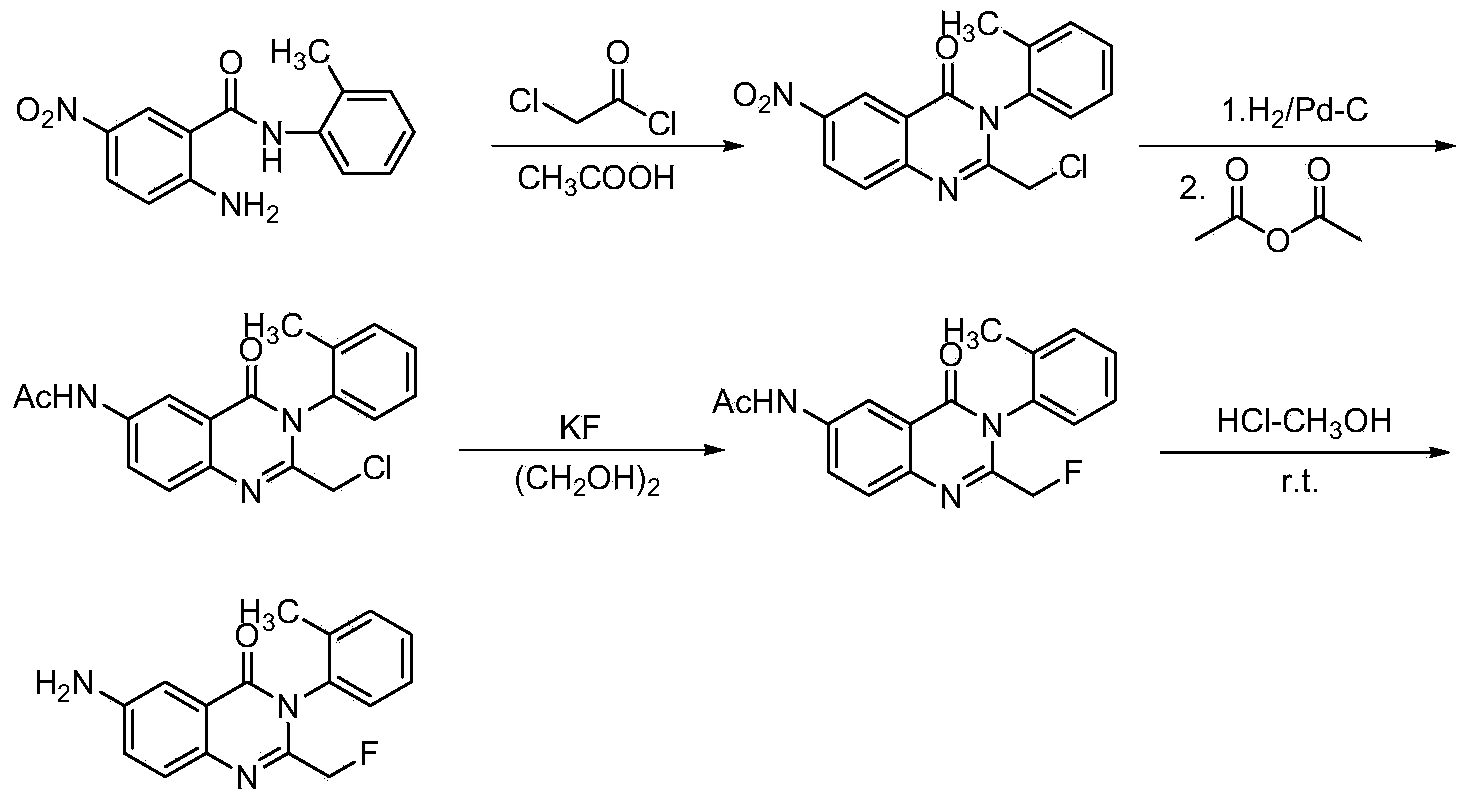
Afloqualone preparation method
A technology for fluoroquinone and naphthoquinone, which is applied in the field of preparation of fluoroquinone, can solve the problems of low total yield, increase fluorine exchange reaction, increase upper protection and deprotection reaction, etc., and achieves reduction of production cost, The effect of increasing conversion rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of 5-Nitroisatoic Anhydride 2
[0035] Add concentrated sulfuric acid (100mL) into a 250mL three-necked flask, slowly add isatoic anhydride 1 (30.0g, 0.18mol) under mechanical stirring at 0-10°C, stir and dissolve, then add 65% concentrated nitric acid (21.4g, 0.22 mol), maintain the temperature at 0-10°C, drop the addition for 1 hour, keep warm and continue the reaction for 1 hour, stop stirring, slowly pour the reaction solution into 300mL ice-water mixture, stir for 20 minutes, the solid is fully separated out, filtered, washed with water, and dried to obtain 5-nitroisatoic anhydride 2 (37.5 g, yield: 97.9%). m.p.264.8-265.5°C; the purity measured by HPLC is 98.4% (the mobile phase is acetonitrile: 20mmol / L KH 2 PO 4 Aqueous solution (pH3.25)=30:70, V / V).
Embodiment 2
[0037] Synthesis of 5-Nitroisatoic Anhydride 2
[0038] Add concentrated sulfuric acid (100mL) into a 250mL three-necked flask, slowly add isatoic anhydride 1 (30.0g, 0.18mol) at 0-10°C under mechanical stirring, stir and dissolve, then add dropwise 98% fuming nitric acid (14.2g, 0.22mol), maintain the temperature at 0-5°C, add dropwise for 1h, keep warm and continue to react for 1h, stop stirring, slowly pour the reaction solution into 300mL ice-water mixture, stir for 20min, the solid is fully separated out, filtered, washed with water, and dried 5-nitroisatoic anhydride 2 (37.0 g, yield: 96.6%) was obtained. m.p.263.8-265.3°C; the purity measured by HPLC is 97.2% (the mobile phase is acetonitrile: 20mmol / L KH 2 PO 4 Aqueous solution (pH3.25)=30:70, V / V).
Embodiment 3
[0040] Synthesis of 5-Acetamidoisatoic Anhydride 3
[0041] In a 500mL autoclave, add 5-nitroisatoic anhydride 2 (20.0g, 96.0mmol), tetrahydrofuran (200mL), Raney nickel (1.00g, purchased from Aladdin Reagents (Shanghai) Co., Ltd., product number R111435, The specification is 500g, 20-40 mesh, and the Raney nickel used in other examples of this specification is the same), first replace the air with nitrogen three times, check the air tightness, then replace the nitrogen with hydrogen three times, and maintain the pressure at 2.0Mpa with hydrogen , react at 60°C for 2h, stop the reaction, filter out Raney nickel, add acetic anhydride (9.79g, 96.0mmol) to the filtrate at room temperature, stir and react for 1h, then concentrate under reduced pressure and dry to obtain 5-acetamido Isatoic anhydride 3 (20.1 g, yield: 95.0%). m.p.215.1-216.0°C; the purity measured by HPLC is 98.0% (the mobile phase is acetonitrile: 20mmol / L KH 2 PO 4 Aqueous solution (pH3.25)=50:50, V / V).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com