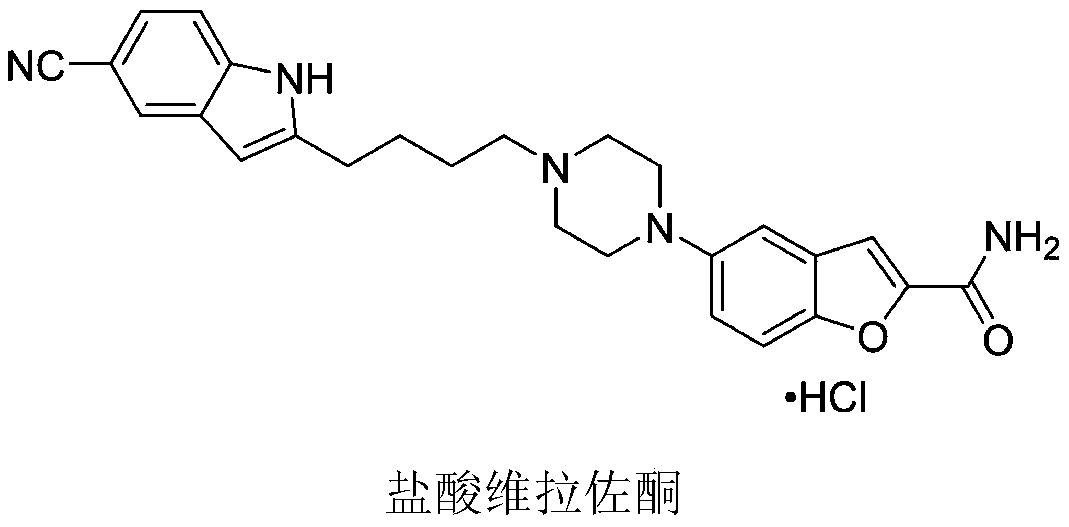
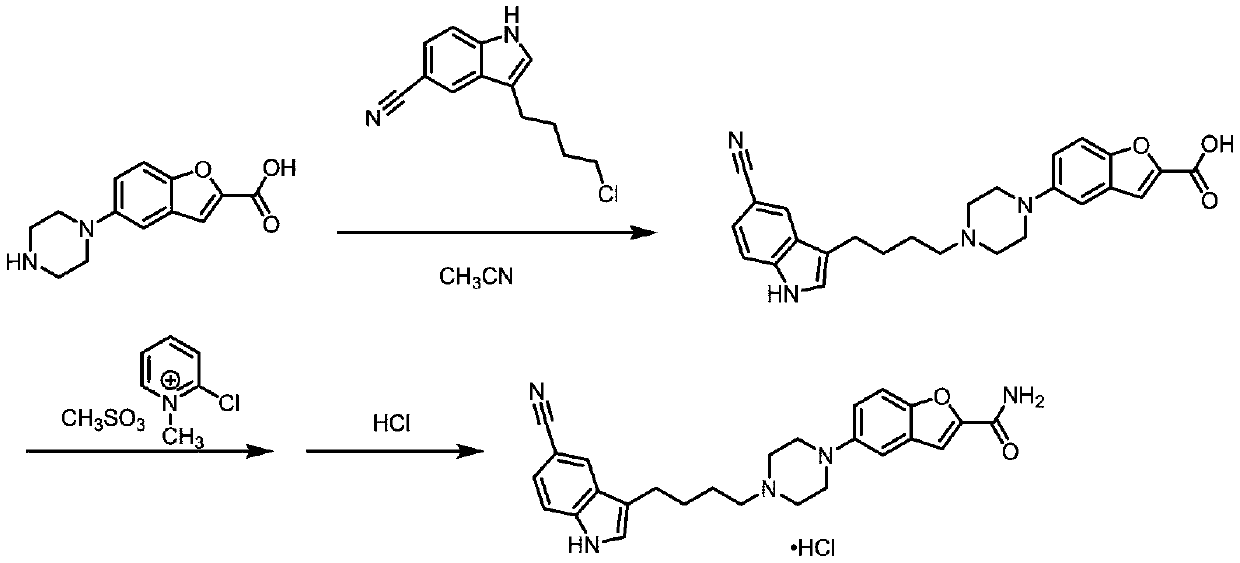
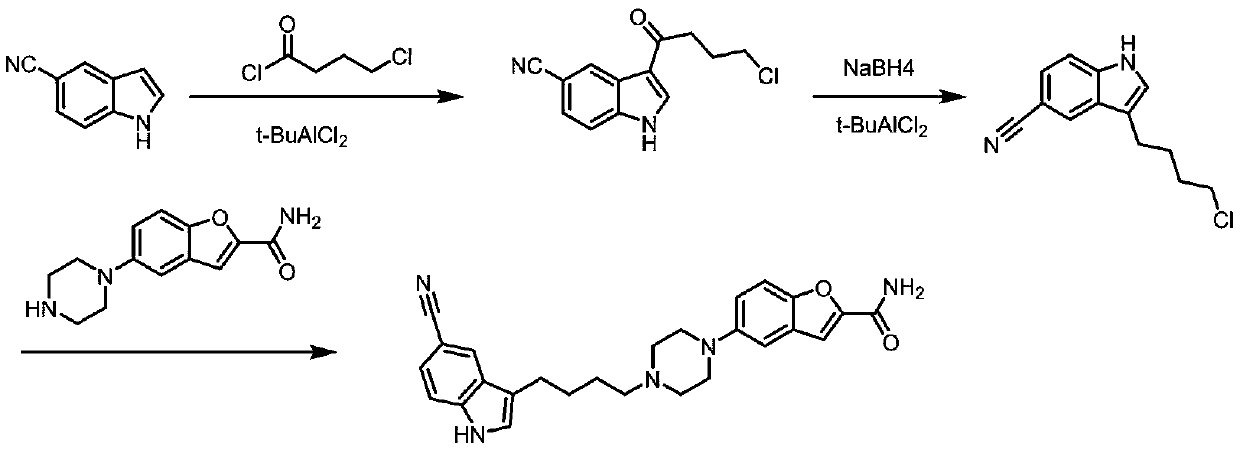
Method for preparing vilazodone intermediate and vilazodone drug by using cheap metal copper
A technology for vilazodone and intermediates, which is applied in the field of preparing vilazodone medicines, can solve the problems of difficult post-processing, long routes, and high preparation costs, and achieves good product purity and content, high atom economy, and good purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1: Add ethyl 5-iodobenzofurancarboxylate (15.8g, 0.05mol), lithium hydroxide (5.99g, 0.25mol) and ammonia water (35.0g, 1.0mol) into a 500mL single-necked bottle, and stir at 50°C After 2 hours, TLC monitored the end point of the reaction, suction filtered, washed with water, and dried to obtain 13.6 g of white solid 5-iodobenzofuran-2-carboxamide, with a yield of 94.4% and a content of 99.5%. The data characterization of the 5-iodobenzofuran-2-carboxamide prepared in embodiment 1: 1 H NMR (400 MHz, CDCl 3 ):δ8.20(s,1H),8.00(d,1H),7.77(s,1H),7.59(m,2H),7.51(s,1H). 13 C NMR (100MHz, CDCl 3 ,ppm)δ159.00,152.74,150.15,129.15,129.05,124.90, 115.57,113.64,108.72.MS(ESI):240.0(100),242.0(98)([M+H] + ).
Embodiment 2
[0078] Example 2: Add ethyl 5-iodobenzofurancarboxylate (15.8g, 0.05mol), sodium hydroxide (10.0g, 0.25mol) and ammonia water (35.0g, 1.0mol) into a 500mL single-necked bottle, and stir at 50°C After 2 hours, TLC monitored the end point of the reaction, suction filtered, washed with water, and dried to obtain 14.0 g of white solid 5-iodobenzofuran-2-carboxamide, with a yield of 97.2%.
Embodiment 3
[0079] Example 3: Add ethyl 5-iodobenzofurancarboxylate (15.8g, 0.05mol), sodium methoxide (13.5g, 0.25mol) and ammonia water (35.0g, 1.0mol) into a 500mL single-necked bottle, and stir at room temperature for 2 hours , TLC to monitor the reaction end point, suction filtration, washing with water, and drying to obtain 13.5 g of white solid 5-iodobenzofuran-2-carboxamide, with a yield of 93.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com