A kind of preparation method of 2-nitro-4-methylsulfonyl benzoic acid
A technology of thiamphenicol benzoic acid and thiamphenicol toluene, which is applied in the field of preparation of 2-nitro-4-thiamphenicol benzoic acid, which can solve the problems of heavy metal pollution, difficulty in methyl oxidation, and difficult availability of raw materials, etc. problem, to achieve the effect of stable and efficient catalytic oxidation process, easy to realize industrial production, and green and environmental protection in the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
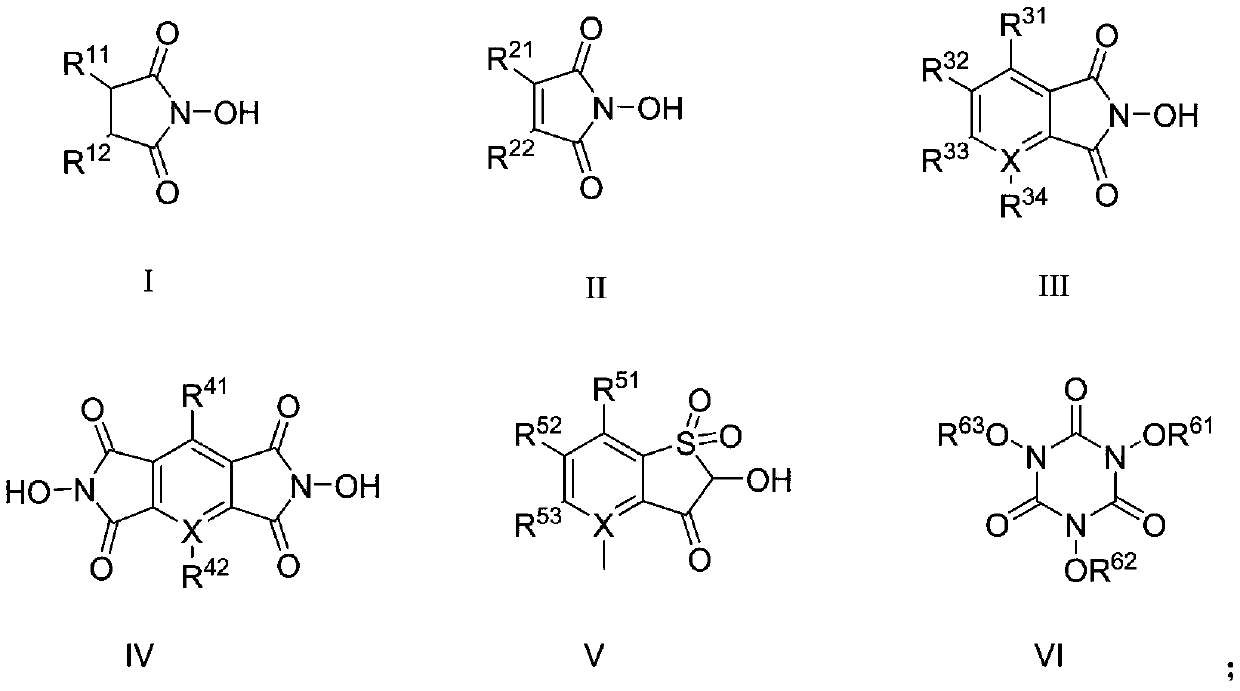
[0022] In a 500mL autoclave, add 21.5g 2-nitro-4-thiamphenicol toluene, add 200g glacial acetic acid, add 1.5g N-hydroxyphthalimide, 0.5g fuming nitric acid, and feed air into the kettle The internal pressure was 1.0 MPa, stirring and heating were started, the reaction temperature was controlled at 110°C, and the reaction was carried out for 8 hours. After the reaction was completed, it was cooled to room temperature, the solution was filtered, and the filter cake was washed with 150 mL of water. Then the filter cake was dissolved with 0.1M sodium hydroxide, the insoluble matter was filtered off, the obtained filtrate was acidified with hydrochloric acid, and after drying, 11.9 g of light yellow solid was obtained. The melting point is 213.3-214.7°C. After HPLC analysis, the purity of 2-nitro-4-thiamphenicol benzoic acid is 98.8%, and the yield is 48%.
Embodiment 2
[0024] Add 21.5g of 2-nitro-4-thiamphenicol toluene to a 500mL autoclave, add 200g of glacial acetic acid, add 3g of N,N'-dihydroxypyromellitic imide, 0.5g of fuming nitric acid, and let air in When the pressure in the kettle is 4.0MPa, start stirring and heating, and control the reaction temperature at 110°C. Reaction 10h. Cool to room temperature after the reaction, filter the solution, and wash the filter cake with 150 mL of water. Then the filter cake was dissolved with 0.1M sodium hydroxide, the insoluble matter was filtered off, the obtained filtrate was acidified with hydrochloric acid, and after drying, 17.1 g of light yellow solid was obtained. The melting point is 212.7-214.1°C. After HPLC analysis, the purity of 2-nitro-4-thiamphenicol benzoic acid is 98.3%, and the yield is 68.6%.
Embodiment 3
[0026] Add 21.5g of 2-nitro-4-thiamphenicol toluene to a 500mL autoclave, add 200g of glacial acetic acid, add 1.5g of N,N'-dihydroxypyromellitic imide, 0.2g of fuming nitric acid, and Oxygen until the pressure inside the kettle is 1.5MPa, start stirring and heating, and control the reaction temperature at 140°C. Reaction 3h. Cool to room temperature after the reaction, filter the solution, and wash the filter cake with 150 mL of water. Then the filter cake was dissolved with 0.1M sodium hydroxide, the insoluble matter was filtered off, the obtained filtrate was acidified with hydrochloric acid, and after drying, 19.5 g of light yellow solid was obtained. The melting point is 212.5-213.8°C. After HPLC analysis, the purity of 2-nitro-4-thiamphenicol benzoic acid is 98.6%, and the yield is 78.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com