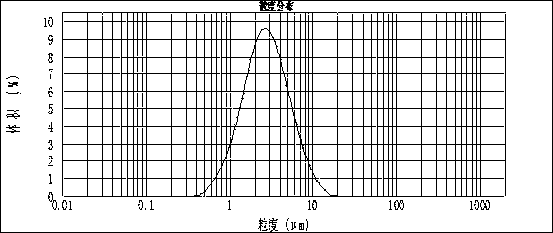
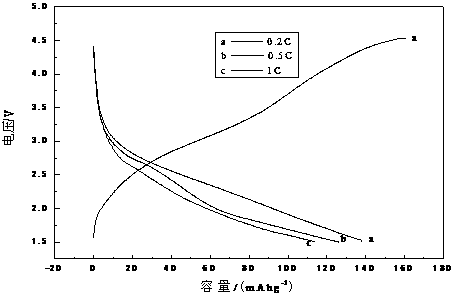
Sol precipitation preparation method for carbon-coated lithium iron silicate cathode material
A technology of lithium iron silicate and positive electrode materials, applied in the direction of electrode manufacturing, battery electrodes, electrical components, etc., can solve problems such as difficult industrial production, unsuitable for industrial production, complex operating conditions, etc., and achieve good electrochemical performance and easy industrialization The effect of simple production and process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Add silicon tetrachloride to absolute ethanol to form a solution with a concentration of 0.1mol / L, and calibrate its concentration for later use;
[0029] (2) Take out 20L of the above solution and place it in a reaction vessel, add water dropwise while stirring to promote its hydrolysis into a transparent sol;
[0030] (3) In the above sol, according to Li:Fe:Si=2:1:1 (molar ratio), add the aqueous solution made of lithium acetate and the aqueous solution made of ferrous acetate, and then add the solution made of sodium hydroxide. The aqueous solution forms a light green emulsion. At this time, the alkali concentration of the reaction system is controlled at 5mol / L, and the aging reaction is carried out in a hot water bath at 90°C and stirred for 8 hours;
[0031] (4) Washing the aged light green emulsion with an inorganic ceramic membrane to obtain a slurry liquid that removes impurity ions brought in by the raw material;
[0032] (5) Add the aqueous solution mad...
Embodiment 2
[0038] (1) Add silicon tetrachloride to absolute ethanol to form a solution with a concentration of 1.5mol / L, and calibrate its concentration for later use;
[0039] (2) Take out 20L of the above solution and place it in a reaction vessel, add water dropwise while stirring to promote its hydrolysis into a transparent sol;
[0040] (3) In the above sol, according to Li:Fe:Si=2:1:1 (molar ratio), add the aqueous solution made of lithium nitrate and the aqueous solution made of ferrous nitrate, and then add the solution made of potassium hydroxide. The aqueous solution forms a light green emulsion. At this time, the alkali concentration of the reaction system is controlled at 8mol / L, and the aging reaction is carried out in a hot water bath at 80°C and stirred for 6.5h;
[0041] (4) Washing the aged light green emulsion with an inorganic ceramic membrane to obtain a slurry liquid that removes impurity ions brought in by the raw material;
[0042] (5) Add the aqueous solution mad...
Embodiment 3
[0047] (1) Add silicon tetrachloride to absolute ethanol to form a solution with a concentration of 3mol / L, and calibrate its concentration for later use;
[0048] (2) Take out 20L of the above solution and place it in a reaction vessel, add water dropwise while stirring to promote its hydrolysis into a transparent sol;
[0049] (3) In the above sol, according to Li:Fe:Si=2:1:1 (molar ratio), add the aqueous solution made of lithium hydroxide and the aqueous solution made of ferrous chloride, and then add potassium hydroxide to make it The resulting aqueous solution generates a light green emulsion. At this time, the alkali concentration of the reaction system is controlled at 10mol / L, and the aging reaction is carried out in a 70°C hot water bath and stirred for 5h;
[0050] (4) Washing the aged light green emulsion with an inorganic ceramic membrane to obtain a slurry liquid that removes impurity ions brought in by the raw material;
[0051] (5) Add the aqueous solution mad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge specific capacity | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com