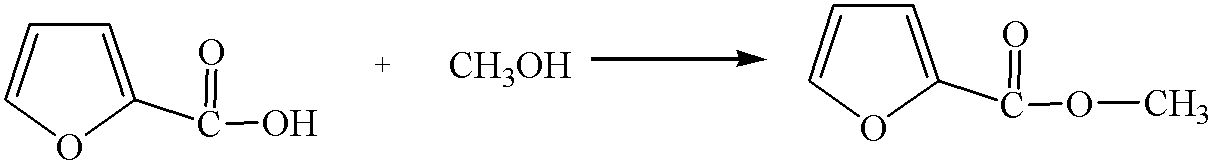Biodiesel fuel and preparation method thereof
A bio-based, diesel technology, used in the preparation of organic compounds, biofuels, and carboxylate esters, etc., can solve the problems of unrealizable development routes, poor low-temperature fluidity and oxidation stability, and high raw material costs, and achieve great economic value. and social benefits, reducing pollution and saving mineral resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
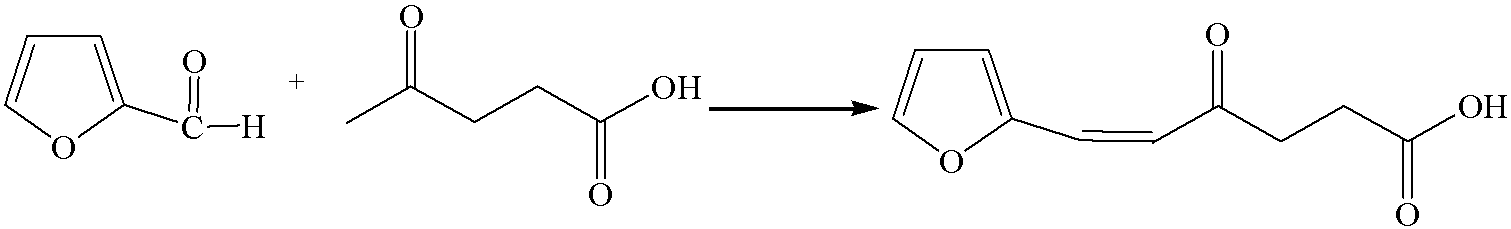
[0032] 1. Add the prepared saturated sodium chloride solution of sodium hydroxide to the reaction kettle, and then add levulinic acid and furfural with a molar ratio of 1:1 in sequence. Turn on the stirring, raise the temperature to 35°C at 0.5°C / min, and the pressure is 0.2MPa. After 30 minutes of reaction, the yield of 6-furfurylidene levulinic acid is 38%.
[0033] 2. Select the mixed alcohols as methanol, butanol and octanol, and the molar ratio is 4:2:1. Add the mixed alcohol and 6-furfurylidene levulinic acid with a molar ratio of 4:1 into the esterification reaction kettle. The esterification reaction temperature is 240° C., the relative pressure is 2 MPa, and the reaction time is 60 minutes. The conversion rate of 6-furfurylidene levulinic acid is 100%, and the yield of methyl 6-furfurylidene levulinate, butyl 6-furfurylidene levulinate and octyl 6-furfurylidene levulinate in the product The rate is 95%.
[0034] 3. Prepare copper-silver-cerium catalyst by co-precip...
Embodiment 2
[0040] 1. Add the prepared saturated sodium chloride solution of sodium hydroxide to the reaction kettle, and then add levulinic acid and furfural with a molar ratio of 1:2 in sequence. Start the stirring, raise the temperature to 35°C at 0.5°C / min, and the pressure is 0.4MPa. After 30 minutes of reaction, the yield of 6-furfurylidene levulinic acid is 43%.
[0041] 2. Select the mixed alcohols as butanol, pentanol and octanol, and the molar ratio is 4:2:1. Add the mixed alcohol and 6-furfurylidene levulinic acid with a molar ratio of 2:1 into the esterification reaction kettle. The esterification reaction temperature is 240° C., the relative pressure is 2 MPa, and the reaction time is 60 minutes. The conversion rate of 6-furfurylidene levulinic acid is 100%, and the yield of 6-furfurylidene levulinate butyl, 6-furfurylidene levulinate pentyl, 6-furfurylidene octyl levulinate in the product is 100%. The rate is 92%.
Embodiment 3
[0048] 1. Add the prepared saturated sodium chloride solution of sodium hydroxide to the reaction kettle, and then add levulinic acid and furfural with a molar ratio of 1:2 in sequence. Start the stirring, raise the temperature to 40°C at 0.5°C / min, and the pressure is 0.6MPa. After 30 minutes of reaction, the yield of 6-furfurylidene levulinic acid is 45%.
[0049] 2. Select the mixed alcohols as methanol, isobutanol, cyclopropanol, octanol, and the molar ratio is 4:4:1:1. Add the mixed alcohol and 6-furfurylidene levulinic acid with a molar ratio of 8:1 into the esterification reaction kettle. The esterification reaction temperature is 280° C., the relative pressure is 4 MPa, and the reaction time is 40 minutes. The conversion rate of 6-furfurylidene levulinic acid is 100%, and the yield of 6-furfurylidene levulinate butyl, 6-furfurylidene levulinate pentyl, 6-furfurylidene octyl levulinate in the product is 100%. The rate is 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com