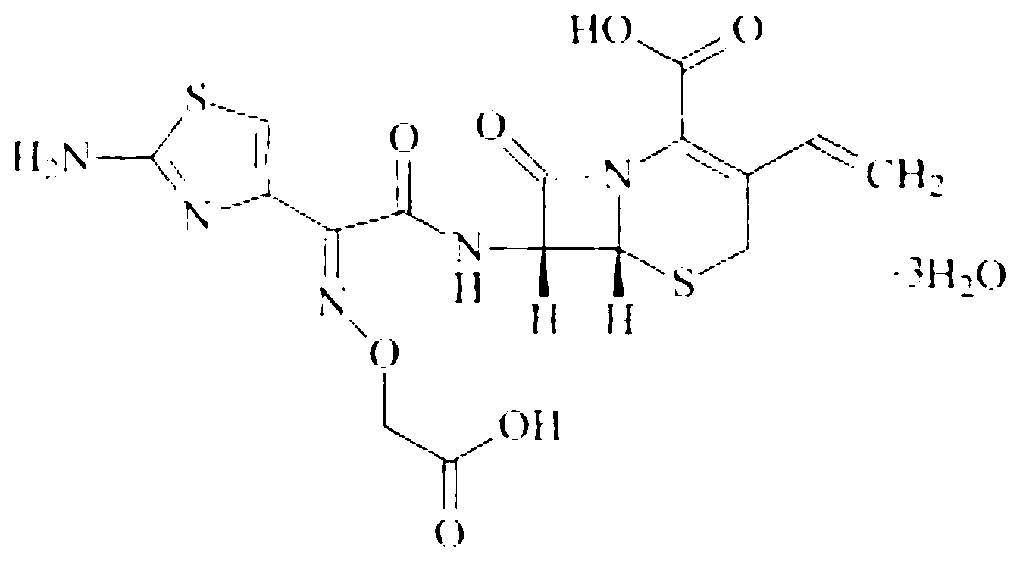
Cefixime dry suspension and preparation method thereof
A technology of cefixime and dry suspension, applied in the field of medicine, can solve the problems of unfavorable application in production, low bioavailability, unpleasant drug dissolution, etc., and achieves improved bioavailability, improved drug stability, and preparation stability. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027]
[0028] Preparation Process:
[0029] (1) Weigh cefixime for the prescription amount, add it to saturated caprylic triglyceride under stirring condition to dissolve, then add polyoxyethylene castor oil to the oil solution, stir to obtain a clear solution;
[0030] (2) The prescription amount weighs povidone and pregelatinized starch, and mixes them evenly to obtain a mixture;
[0031] (3) Adsorb the solution of step (1) with the mixture of step (2), and stir evenly;
[0032] (4) Subpackage on a granule packaging machine to obtain Cefixime dry suspension.
Embodiment 2
[0034]
[0035] Preparation Process:
[0036] (1) Weigh cefixime for the prescription amount, add it into saturated capric triglyceride under stirring condition to dissolve, then add polyoxyethylene castor oil into the oil solution, stir to obtain a clear solution;
[0037] (2) Weigh the lactose and sodium carboxymethyl cellulose for the prescription amount, mix them evenly, and obtain the mixture;
[0038] (3) Adsorb the solution of step (1) with the mixture of step (2), and stir evenly;
[0039](4) Subpackage on a granule packaging machine to obtain Cefixime dry suspension.
Embodiment 3
[0041]
[0042] Preparation Process:
[0043] (1) The prescription quantity weighs cefixime, and under stirring conditions, add caprylic acid to capric acid molar ratio of 1:1 saturated caprylic acid-capric acid mixed triglyceride to dissolve, and then add polyoxygen to the oil solution. Ethylene castor oil, stirred to obtain a clear solution;
[0044] (2) Pre-gelatinized starch and hydroxypropyl cellulose are weighed for the prescription amount, and mixed evenly to obtain a mixture;
[0045] (3) Adsorb the solution of step (1) with the mixture of step (2), and stir evenly;
[0046] (4) Subpackage on a granule packaging machine to obtain Cefixime dry suspension.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com