PEG modified polyethylene imine derivative and preparation method thereof
A technology of polyethyleneimine and polyethylene glycol, which is applied in the direction of medical preparations of non-active ingredients, pharmaceutical formulas, genetic material components, etc., can solve the problem of reducing non-specific reactions, reducing the aggregation of PEI/DNA complexes, and transfection Reduced activity and other issues, to achieve the effect of reducing cytotoxicity, improving transfection activity, and reducing cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation of the PEI derivative (PEG-Bu) of embodiment 1 PEG modification
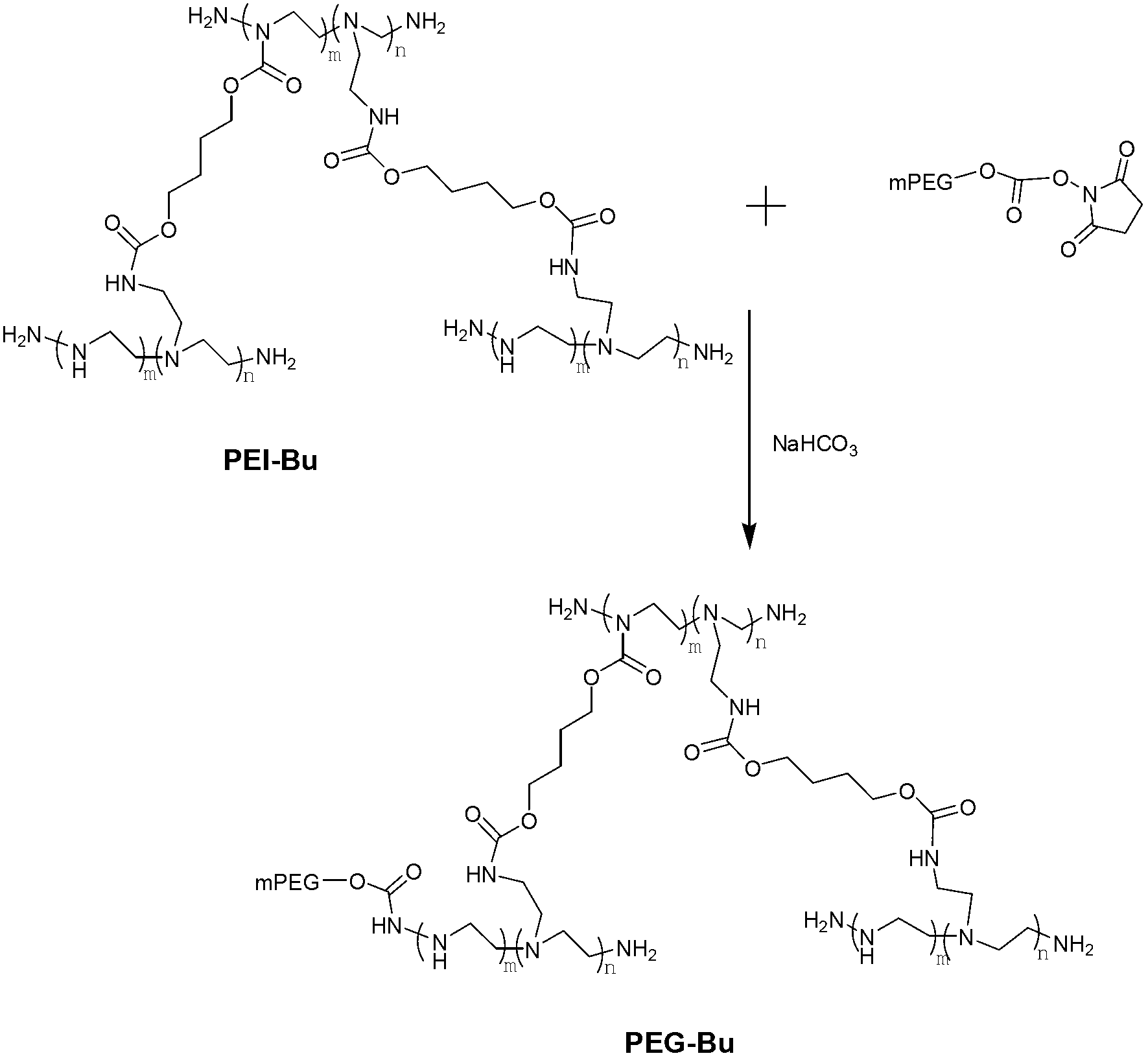
[0051] figure 1 It is a schematic diagram of the synthetic route of PEG-modified PEI derivatives, including the following steps:
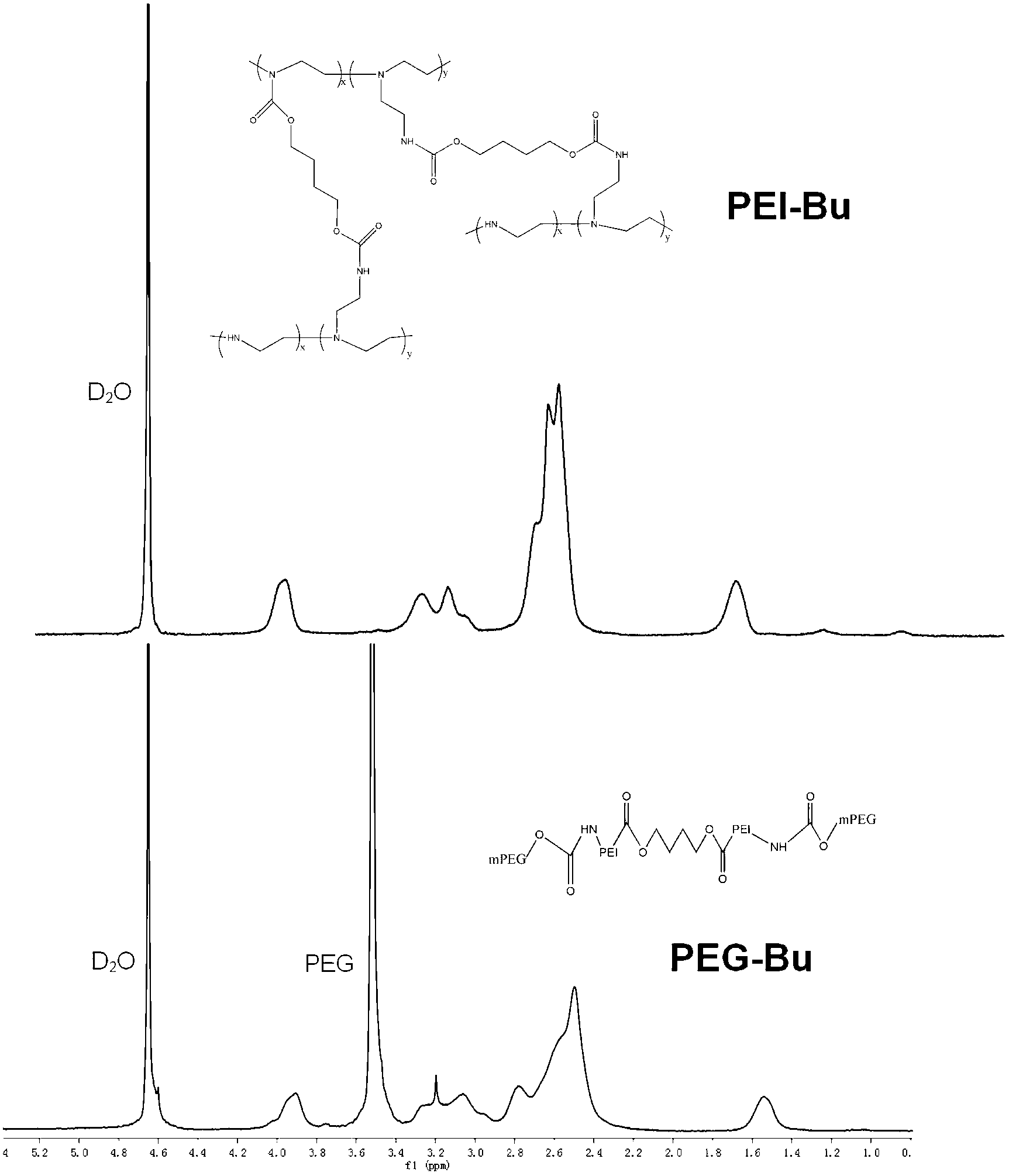
[0052] (a) prepare PEI-Bu according to the method described in CN102443169A, carry out infrared and nuclear magnetic detection, the results are respectively as follows figure 1 with figure 2 As shown, the results showed that PEI-Bu was obtained;
[0053] (b) Dissolve PEI-Bu in sodium bicarbonate solution, then stir and shake at room temperature with methoxypolyethylene glycol-succinimide carbonate (mPEG-Sc) in a molar ratio of 3:1 Or shock reaction for 4 hours.
[0054] (c) Separation and purification, the product is dissolved with a small amount of ultrapure water and then placed in a dialysis bag with a molecular weight cut-off of 3500Da for dialysis for 48 hours. After the dialysis, the product is filtered with a 0.22 μm microporous membrane, and then t...
Embodiment 2
[0062] Preparation of the complex (Polyplex) synthesized by embodiment 2 PEG-Bu and plasmid
[0063] Weigh quantitative polymer PEG-Bu, add ultrapure water to make a 2mg / mL solution, then filter it with a 0.22μm sterile filter head, and dilute the concentration of luciferase (pGL3-Control) plasmid to 1mg / mL;
[0064] Configure complex solutions with different mass ratios, keep the concentration of the plasmid solution constant, and then dilute the concentration of the polymer solution according to the mass ratio of the polymer (PEG-Bu) to the plasmid, and keep the diluted polymer solution and plasmid solution Finally, the polymer solution was quickly added to the plasmid solution and mixed, and incubated at room temperature for 30-120 minutes, so that a series of complexes with a mass ratio were obtained, which could be used for further determination of physical and chemical properties.
[0065] The complex agarose gel electrophoresis: Prepare agarose solution with a mass ra...
Embodiment 3
[0073] The cytotoxicity test of embodiment 3PEG-Bu
[0074] Inoculate cells (BRL-3A, Hela), digest the cells, and dilute to a density of 5×10 4 -10×10 4 / mL cell suspension, add 100 μL to each well of a 96-well plate, and incubate overnight.
[0075] Dilute the 2 mg / mL high molecular weight DMEM solution into different concentration gradients with a final volume of 100 μL. The complexes synthesized by different mass ratios of PEG-Bu and plasmid were prepared.
[0076] After taking out the cells, remove the medium with serum, wash with 100 μL of PBS (phosphate) buffer, directly add the prepared polymer DMEM solution or complex to each cell well, add 100 μL to the negative control group Serum-free phenol red-free DMEM. After 4 hours, remove the culture medium and polymer solution, add 100 μL of serum-free phenol red-free medium to each well, and then add 25 μL of MTT solution (3-(4,5-dimethylthiazole-2) -2,5-diphenyltetrazolium bromide salt solution, the solution was prepar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com