Polymeric micelle medicine composition and preparation method thereof
A technology of polymer glue, composition, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Synthesis of monomethoxypolyethylene glycol-polylactic acid block copolymer (mPEG2000-PDLLA2482)
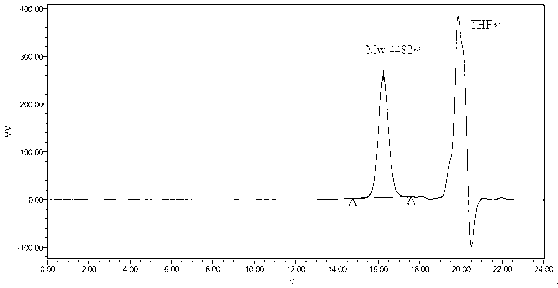
[0061] Take 4 g of monomethoxypolyethylene glycol (Mw 2000), place it at 120 °C for 3 hours in vacuum, add 6 g of lactide monomer and 5 μl of stannous isooctanoate (equivalent to 0.063% of the total amount of monomer) in In the polymerization tube, vacuumize for 30 minutes, seal the tube, and polymerize at 140°C for 6 hours. The polymerized product was dissolved in 20ml of dichloromethane, dropped into pre-cooled excess ether, and the product was precipitated at -20°C. The precipitate was filtered and washed with ether. The operation was repeated three times, and then the filter residue was dried under reduced pressure at room temperature for 48 hours to obtain a block copolymer mPEG-PDLLA with high purity (the yield was about 78%). NMR results see figure 1 , the gel permeation chromatography results of the polymer are shown in figure 2 .
Embodiment 2
[0062] Example 2 Synthesis of monomethoxypolyethylene glycol-polylactic acid block copolymer (mPEG5000-PDLLA3034)
[0063] Take 3 g of monomethoxypolyethylene glycol (Mw 5000), place it at 120 °C for 3 hours in vacuum, add 7 g of lactide monomer and 5 μl of stannous isooctanoate (equivalent to 0.063% of the total amount of monomer) in In the polymerization tube, vacuumize for 30 minutes, seal the tube, and polymerize at 140°C for 6 hours. The polymerized product was dissolved in 20ml of dichloromethane, dropped into pre-cooled excess ether, and the product was precipitated at -20°C. The precipitate was filtered and washed with ether. The operation was repeated three times, and then the filter residue was dried under reduced pressure at room temperature for 48 hours to obtain a block copolymer mPEG-PDLLA with high purity (the yield was about 88%).
[0064] The structure of mPEG2000 / 5000-PDLLA block copolymer is as follows:
[0065]
Embodiment 3
[0066] Example 3 Synthesis of polylactic acid-polyethylene glycol-polylactic acid block copolymer (PDLLA550-PEG2000-PDLLA550)
[0067] Take 6g of polyethylene glycol (Mw 2000), put it under vacuum drying at 120°C for 3 hours, add 4g of lactide monomer, 5μl of stannous isooctanoate (equivalent to 0.063% of the total amount of monomer) in the polymerization tube, Vacuum for 30 minutes, seal the tube, and polymerize at 140°C for 6 hours. The polymerized product was dissolved in 20ml of dichloromethane, dropped into pre-cooled excess ether, and the product was precipitated at -20°C. The precipitate was filtered and washed with ether. The operation was repeated three times, and then the filter residue was dried under reduced pressure at room temperature for 48 hours to obtain the block copolymer PDLLA-PEG-PDLLA (the yield was about 85%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com