Preparation of gold nanoparticle coated with folic acid-modified pegylated dendrimer
A dendrimer, PEGylation technology, used in the preparation of X-ray contrast agents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] (1) Dilute 20 mg of folic acid with 20 mL of dimethyl sulfoxide solution and activate with 8.69 mg of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) 3h, and then dropwise added polyethylene glycol (NH 2 -PEG-COOH), after reacting for 70~80h, the reaction product was dialyzed in the buffer solution with a dialysis membrane for 24h, and then dialyzed with ultrapure water for 48h, and finally the purified product was freeze-dried to obtain a folic acid-modified polyethylene glycol solid Product (FA-PEG-COOH).
[0058] (2) Dissolve dendrimer with a dry weight of 30 mg in 15 mL of DMSO solution, then take 22.49 mg of the solid obtained in (1), dissolve in 5 mL of DMSO solution and activate with EDC.HCl Afterwards, add the dendrimer solution dropwise, react for 72 hours, and then add polyethylene glycol monomethyl ether (mPEG-COOH) with a dry weight of 34.80 mg activated with EDC.HCl and one end of which is a carboxyl group. The dialysis membrane was d...
Embodiment 2
[0061] (1) Dilute 30 mg of folic acid with 20 mL of dimethyl sulfoxide solution and activate with 13.03 mg of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) 3h, and then dropwise added polyethylene glycol (NH 2 -PEG-COOH), after reacting for 70~80h, the reaction product was dialyzed in the buffer solution with a dialysis membrane for 24h, and then dialyzed with ultrapure water for 48h, and finally the purified product was freeze-dried to obtain a folic acid-modified polyethylene glycol solid Product (FA-PEG-COOH).
[0062] (2) Dissolve dendrimer with a dry weight of 20 mg in 15 mL of DMSO solution, then take 14.99 mg of the solid obtained in (1), dissolve in 5 mL of DMSO solution and activate with EDC·HCl Afterwards, add the dendrimer solution dropwise, react for 72 hours, and then add polyethylene glycol monomethyl ether (mPEG-COOH) with a dry weight of 23.19 mg activated by EDC·HCl with a carboxyl group at one end, and react for 72 hours. The dialysis...
Embodiment 3
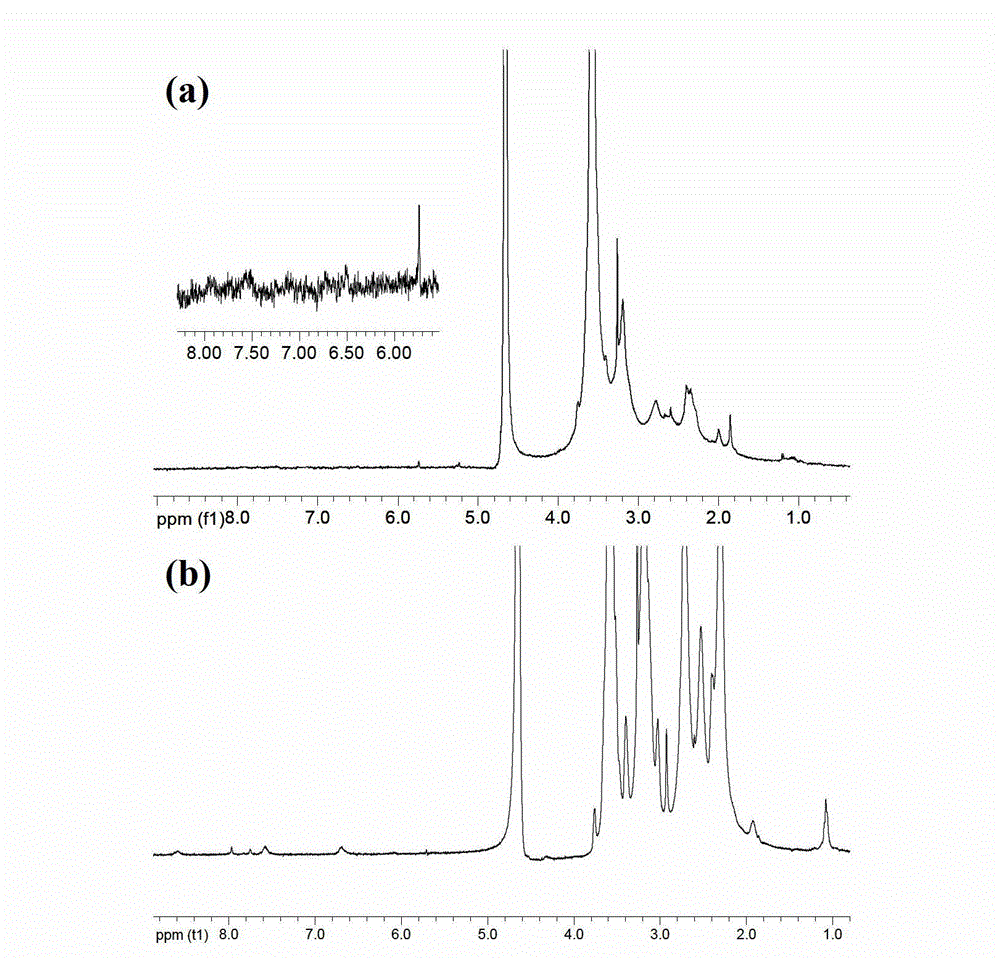
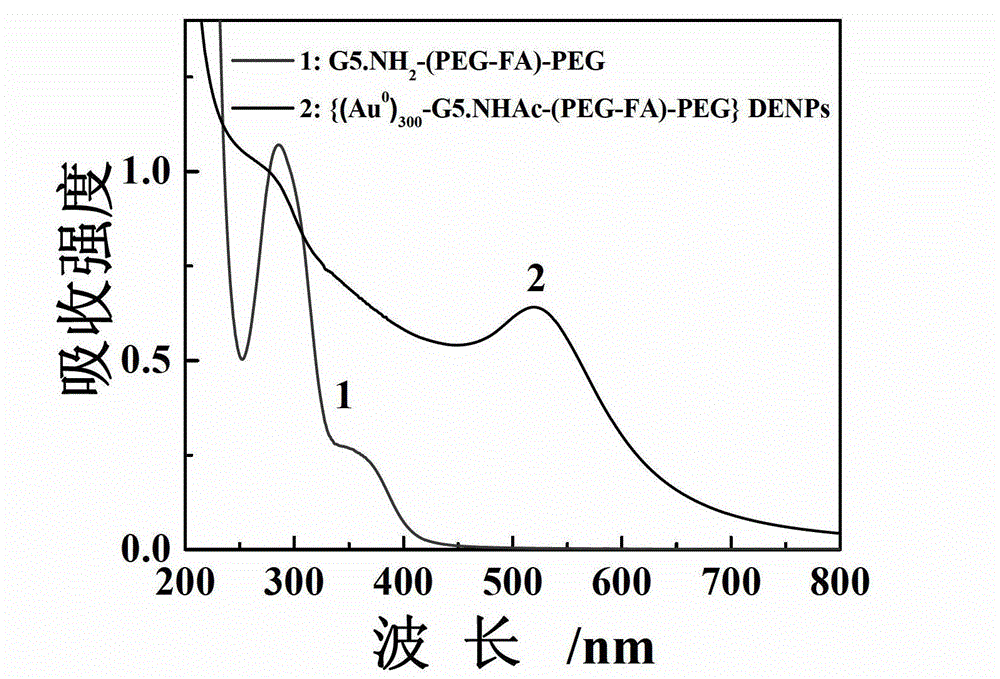
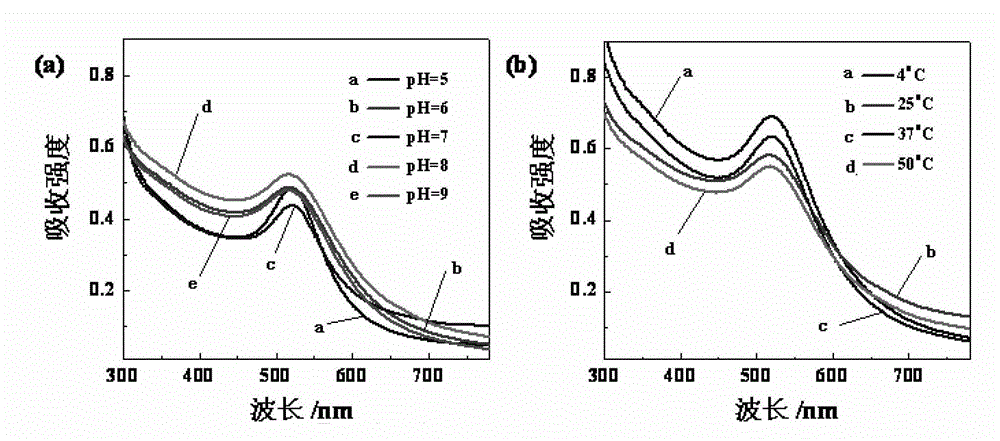
[0064] The G5.NH prepared by the embodiment 1 that got 2 -(PEG-FA)-mPEG, dissolve it with 20mL methanol or water, then add chloroauric acid solution (10mg / mL), stir at room temperature for 30min, then add ice NaBH 4 solution (43.88mg, CH 3 OH:H 2 O (volume ratio) = 2:1), stirred and reacted at room temperature for 2 hours, added 88.9 μL triethylamine to the reaction system, stirred and mixed for 30 minutes, then added 72.4 μL acetic anhydride to the reaction solution, stirred and reacted at room temperature for 24 hours , then the reaction product was dialyzed in PBS buffer solution and ultrapure water with a dialysis membrane, and finally the purified product was freeze-dried to obtain gold nanoparticles ({(Au 0 ) 300 -G5.NHAc-(PEG-FA)-mPEG}DENPs).
[0065] Described chloroauric acid solution is the HAuCl of 10mg / mL 4 Methanol solution or 10mg / mL HAuCl 4 aqueous solution; the NaBH 4 The solution is NaBH 4 H 2 O / CH 3 OH solution, H 2 O and CH 3 The volume ratio of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com