Human cytomegalo virus immunogen fusion protein as well as preparation method and usage thereof
A technology of human cytomegalovirus and fusion protein, which is applied in the field of human cytomegalovirus immunogen fusion protein and its preparation, can solve the problems of poor effectiveness of human cytomegalovirus vaccine, achieve good clinical application prospects, strong immunogenicity, good immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Construction of embodiment 1pET42b / FljB expression vector
[0071] Take the pET42b plasmid and the nucleotides encoding flagellin FljB, and clone the nucleotides encoding flagellin FljB into the pET42b plasmid with NdeI and BamHI endonucleases to obtain the pET42b / FljB vector.
[0072] Ligation transformation: The recovered target fragment was ligated with the pMD19-T simple vector at a molar ratio of 3:1 at 16°C for 1 hour, and then transformed into Escherichia coli TOP10 competent cells (CaCl 2 prepared by method). The specific method for transforming E. coli competent cells with the linker: thaw the TOP10 competent cells on ice; add the ligation reaction (4 μl), rotate gently, and then incubate on ice for 5 minutes; place the test tube in a water bath at 42 Heat shock at °C for 30 seconds; cool the tube on ice for at least 2 minutes; add SOC medium (400 μl) to the cells and shake at 37°C for 1 hour.
[0073] Screening: Use two LB agar kanamycin (50 μg / ml) culture d...
Embodiment 2
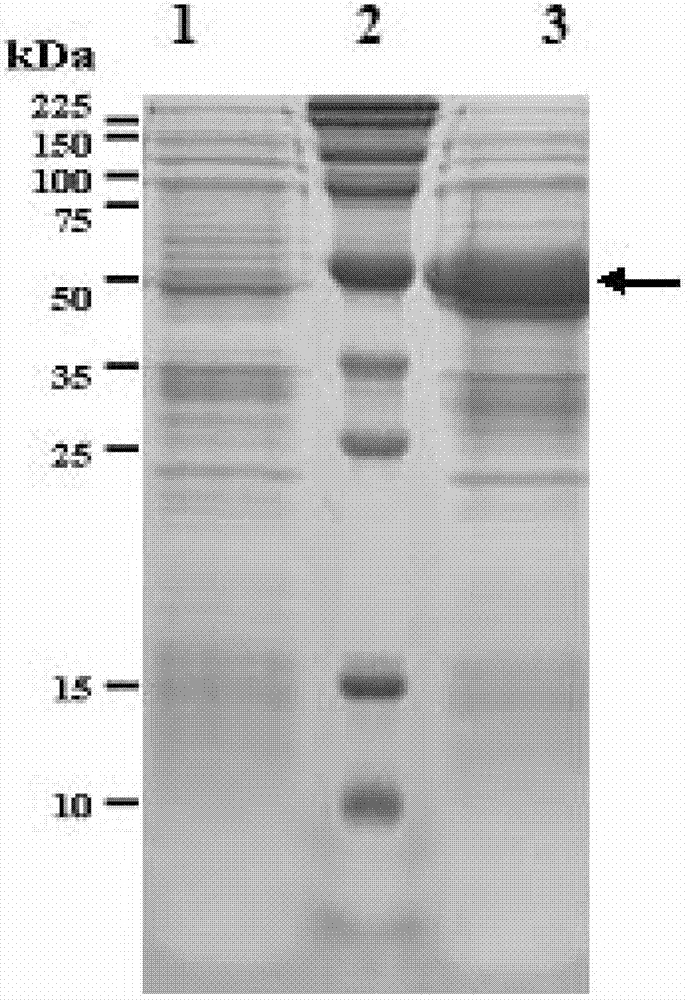
[0075] Example 2FljB-AD1 fusion protein expression vector construction and its expression and purification
[0076] 1. Construction of fusion protein expression vector
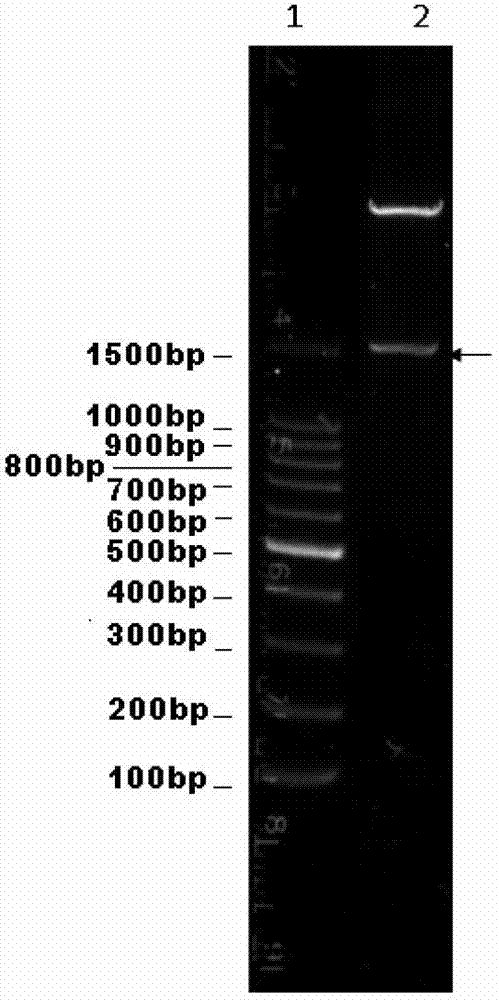
[0077] The pET42b / FljB prepared in Example 1 and the synthesized AD1 nucleotide sequence were subjected to SpeI / KpnI double enzyme digestion respectively. The digested product was subjected to agarose electrophoresis, and the 260bp AD1 and about 7.2kb pET42b / FljB fragments were cut out, purified with Qiagen gel extraction kit, and ligated with pMD19-T simple vector at a molar ratio of 3:1 at 16°C for 1 hour. The ligation product was transformed into Escherichia coli TOP10 competent cells. Positive clones were screened on a kanamycin-resistant LB plate (same as in Example 1), and pET42b / FljB-AD1 was obtained by plasmid extraction and restriction restriction plasmid identification, and the results were as follows figure 2 , indicating that the pET426b / FljB-AD1 vector was successfully constructed, and its stru...
Embodiment 3
[0083] Example 3 Construction of FljB-AD2 fusion protein expression vector and its expression and purification
[0084] 1. Construction of Fusion Protein Expression Vector
[0085] The pET42b / FljB and pUC57 (containing the synthetic AD2 sequence SEQ ID NO: 3) constructed in Example 1 were subjected to SpeI / KpnI double enzyme digestion respectively. The digested product was subjected to agarose electrophoresis, and the 318bp AD2 and about 7.2kb pET42b / FljB fragments were excised and purified with a Qiagen gel extraction kit. The ligation reaction was the same as in Example 1. The ligation product was transformed into Escherichia coli TOP10 competent cells. Positive clones were screened on the kanamycin-resistant LB plate (same as in Example 1), and pET42b / FljB-AD2 was obtained by plasmid extraction and restriction restriction plasmid identification, and the results were as follows Figure 6 , indicating that the pET42b / FljB-AD2 vector was successfully constructed, and its str...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com