Azlocillin sodium medicinal composition for injection and preparation method for azlocillin sodium medicinal composition for injection
An azlocillin and composition technology, which is applied in the field of azlocillin sodium nanoparticle injection for injection and its preparation, can solve the problems of poor drug redissolving and stability, affecting the safety and effectiveness of drug trial, and the like. Wide range of drug storage conditions, excellent stability, and the effect of avoiding degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
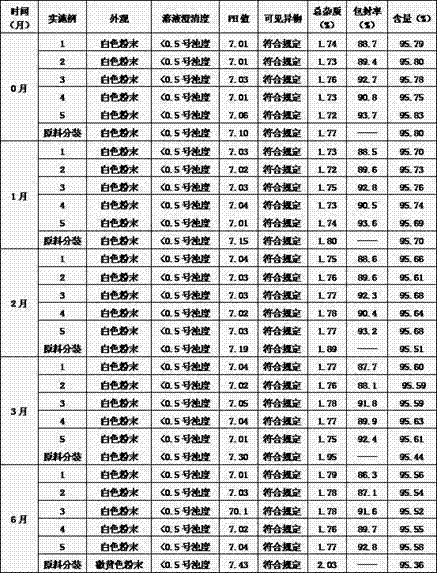
Image
Examples
Embodiment 1
[0057] The preparation of embodiment 1 azlocillin sodium nanoparticle injection
[0058] Prescription 100 bottles
[0059] Azlocillin Sodium 600g
[0060] n-Butyl cyanoacrylate 700g
[0061] Polyethylene glycol 7g
[0062] Glycine 700g
[0063] Preparation Process:
[0064] (1) Add 200g of azlocillin sodium and 7g of polyethylene glycol into 2.4L of water for injection, stir to dissolve, and adjust the pH to 2.0-3.0 with sulfuric acid.
[0065] (2) Add 700g of n-butyl cyanoacrylate to the above solution under stirring, stir at room temperature for 6 hours, add 700g of glycine, continue stirring until completely dissolved, and adjust the pH to 6.0-8.0 with sodium hydroxide.
[0066] (3) Filter the above solution with a 0.22 μm microporous membrane to obtain azlocillin sodium nanoparticle solution.
[0067] (4) Dispense the above solution into sterilized vials, pre-freeze at -40°C to -50°C for 3 hours, freeze-dry with a freeze dryer, seal with a sterile rubber stopper, and...
Embodiment 2
[0068] The preparation of embodiment 2 azlocillin sodium nanoparticle injection
[0069] Prescription 100 bottles
[0070] Azlocillin Sodium 200g
[0071] Propyl cyanoacrylate 600g
[0072] Polyethylene glycol 6g
[0073] Hydrolyzed Gelatin 600g
[0074] Preparation Process:
[0075] (1) Add 200g of azlocillin sodium and 6g of polyethylene glycol into 2L of water for injection, stir to dissolve, and adjust the pH to 2.0-3.0 with formic acid.
[0076] (2) Add 600g of propyl cyanoacrylate to the above solution under stirring, stir at room temperature for 5 hours, add 600g of hydrolyzed gelatin, continue stirring until completely dissolved, and adjust the pH to 6.0-8.0 with sodium bicarbonate.
[0077] (3) Filter the above solution with a 0.22 μm microporous membrane to obtain azlocillin sodium nanoparticle solution.
[0078] (4) Dispense the above solution into sterilized vials, pre-freeze at -40°C to -50°C for 3 hours, freeze-dry with a freeze dryer, seal with a sterile r...
Embodiment 3
[0079] The preparation of embodiment 3 azlocillin sodium nanoparticle injection
[0080] Prescription 100 bottles
[0081] Azlocillin Sodium 200g
[0082] n-Butyl cyanoacrylate 600g
[0083] Poloxamer 6g
[0084] Mannitol 600g
[0085] Preparation Process:
[0086] (1) Add 100g of azlocillin sodium and 5g of poloxamer into 2L of water for injection, stir to dissolve, and adjust the pH to 1.0-2.0 with hydrochloric acid.
[0087] (2) Add 600g of n-butyl cyanoacrylate to the above solution under stirring, stir at room temperature for 5 hours, add 600g of mannitol, continue stirring until completely dissolved, and adjust the pH to 6.0-8.0 with sodium bicarbonate.
[0088] (3) Filter the above solution with a 0.22 μm microporous membrane to obtain azlocillin sodium nanoparticle solution.
[0089] (4) Dispense the above solution into sterilized vials, pre-freeze at -40°C to -50°C for 3 hours, freeze-dry with a freeze dryer, seal with a sterile rubber stopper, and then seal wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com