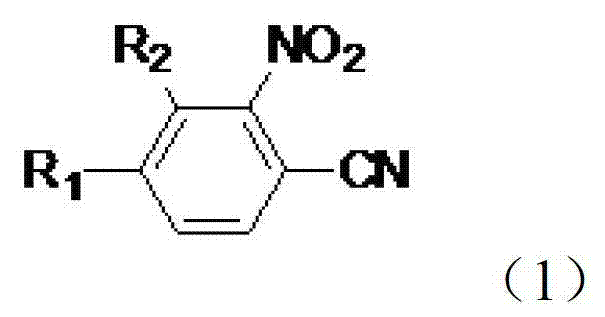
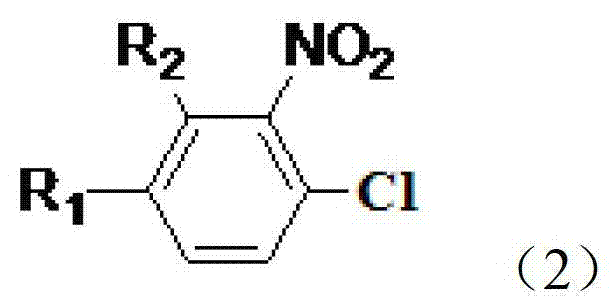
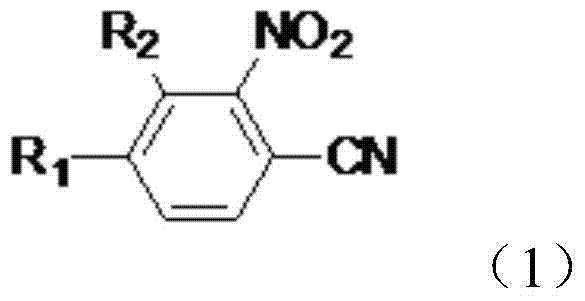
Preparation method of nitrophenylacetonitrile compound
A technology for o-nitrobenzonitrile and compound, which is applied in the field of preparation of o-nitrobenzonitrile compounds, can solve the problems of low price, low reaction yield of ferrocyanide, low toxicity of cyanide reagent, etc. The price of raw materials, the effect of promoting industrial production and expanding the scope of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of 2-nitro-4-trifluoromethylbenzonitrile
[0036] Under the protection of dry nitrogen, add N-methylpyrrolidone (130g), 3-nitro-4-chlorobenzotrifluoride (0.1mol), cuprous chloride (0.05mol), bromide Copper (0.01mol) and anhydrous potassium ferrocyanide (0.02mol) were stirred, heated to 160°C, and stirred for 8 hours. Subsequently, the temperature was lowered to room temperature under the protection of nitrogen, and samples were taken for GC-MS analysis. The analysis results are: the reaction conversion rate is 95.1%, and the reaction selectivity is 91%. Add 600ml of diethyl ether and 150ml of water, stir, separate the organic phase, continue to extract the water phase with diethyl ether, combine the organic phases, dry over anhydrous sodium sulfate, decolorize with activated carbon, and remove the solvent to obtain 18.2g of a yellow solid with a yield of about 84%. The mass spectral data (EI) of 2-nitro-4-trifluoromethylbenzonitrile is: 216(M+)197(M-F)186(...
Embodiment 2
[0056] Preparation of 2-nitro-4-trifluoromethylbenzonitrile
[0057]Under the protection of dry nitrogen, N,N-dimethylacetamide (130g), 3-nitro-4-chlorobenzotrifluoride (0.1mol), cuprous chloride (0.06mol) were added to the reaction flask successively , cuprous bromide (0.03mol), and anhydrous sodium ferrocyanide (0.02mol) were stirred, heated to 170°C, and stirred for 8 hours. Subsequently, the temperature was lowered to room temperature under the protection of nitrogen, and samples were taken for GC-MS analysis. The analysis results are: the reaction conversion rate is 97%, and the reaction selectivity is 93%. Add 600ml of diethyl ether and 150ml of water, stir, separate the organic phase, continue to extract the water phase with diethyl ether, combine the organic phases, dry over anhydrous sodium sulfate, decolorize with activated carbon, and precipitate 18.6g of a yellow solid with a yield of about 86%.
Embodiment 3
[0059] Preparation of 2-nitro-4-trifluoromethylbenzonitrile
[0060] Under the protection of dry nitrogen, N-methylpyrrolidone (130g), 3-nitro-4-chlorobenzotrifluoride (0.1mol), cuprous chloride (0.05mol), bromide Copper (0.02mol) and anhydrous potassium ferrocyanide (0.02mol) were stirred, heated to 170°C, and stirred for 8 hours. Subsequently, the temperature was lowered to room temperature under the protection of nitrogen, and samples were taken for GC-MS analysis. The analysis results are: the reaction conversion rate is 97.4%, and the reaction selectivity is 92.1%. Add 600ml of diethyl ether and 150ml of water, stir, separate the organic phase, continue to extract the water phase with diethyl ether, combine the organic phases, dry over anhydrous sodium sulfate, decolorize with activated carbon, and precipitate 19.2g of a yellow solid with a yield of about 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com