Preparation method and application of benzofuran derivate
A reaction and compound technology, applied in the field of preparation of benzofuran derivatives, can solve problems such as poor solubility and impact on yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
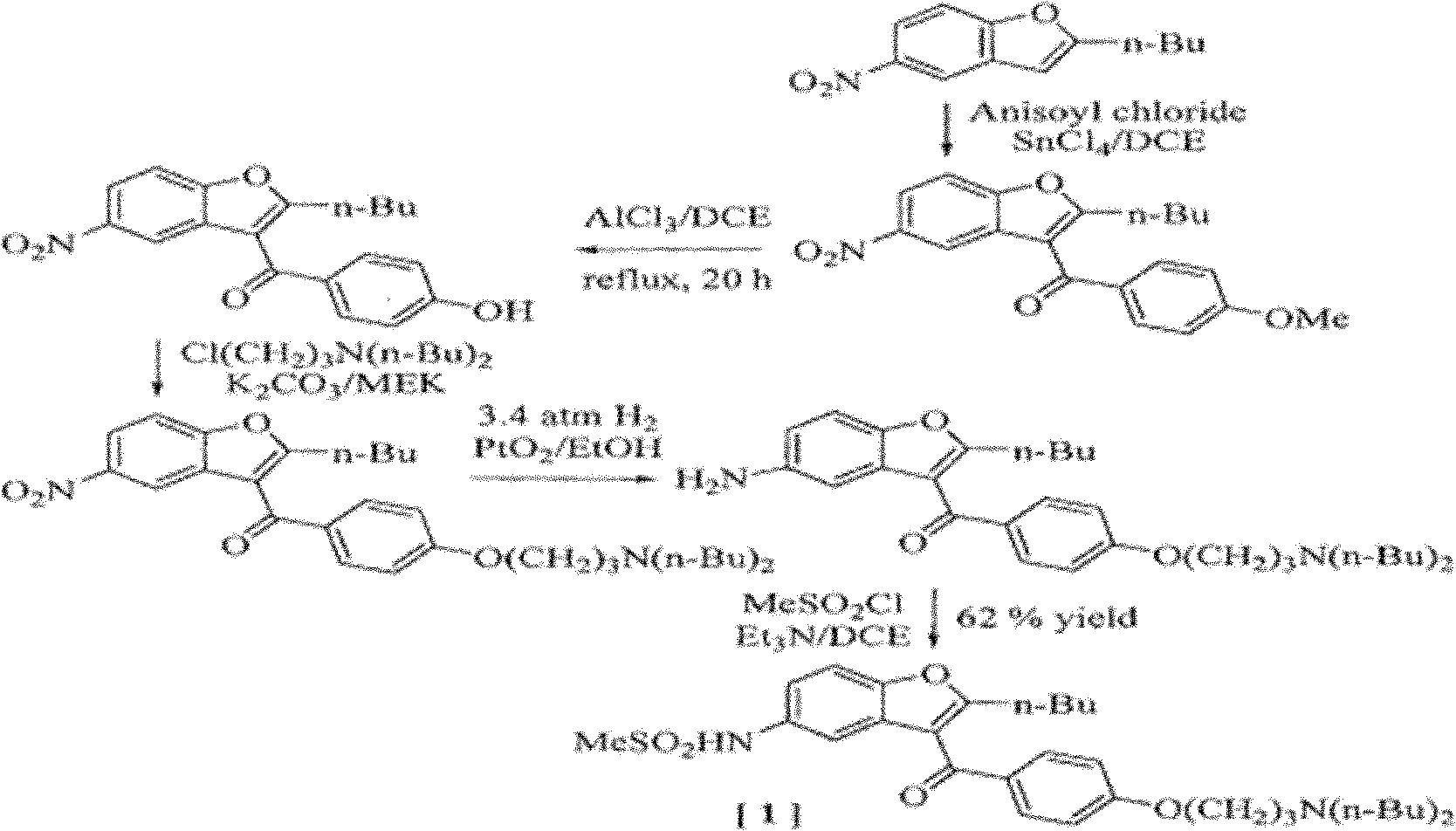
[0047] The compound represented by formula I obtained by the preparation method of the present invention can be used to prepare dronedarone and pharmaceutically acceptable salts thereof. Therefore, the present invention also relates to the compound represented by formula I as an intermediate, which is subjected to N-alkylation reaction with dibutylamine for the final synthesis of dronedarone and its pharmaceutically acceptable salt.
[0048] The N-alkylation reaction can be carried out in polar organic solvents such as dimethylformamide, dimethylsulfoxide, acetonitrile, ethanol, isopropanol, n-butanol, isobutanol, acetone, methyl ethyl ketone, ethyl acetate ester, butyl acetate, ether, or tetrahydrofuran, or non-polar organic solvents such as substituted benzene, n-hexane, cyclohexane, or petroleum ether, or water, or a mixture of the above solvents, or in the presence of no solvent. Among them, dimethylformamide and acetonitrile are preferable.
[0049] The N-alkylation reac...
Embodiment 1
[0068] Step a) the preparation of 4-(3-chloropropoxy) methyl benzoate
[0069] 17.2 g of bromochloropropane, 15.2 g of methyl p-hydroxybenzoate, 15.8 g of anhydrous potassium carbonate and 100 ml of toluene were added to a 250 ml reaction flask, and the mixture was stirred and refluxed for 6 hours. After cooling to room temperature, it was filtered with suction, and the filtrate was concentrated to dryness under reduced pressure to obtain an off-white solid, which was directly used in the next reaction.
[0070] Step b) Preparation of 4-(3-chloropropoxy)benzoic acid
[0071] The above obtained methyl 4-(3-chloropropoxy)benzoate was added to 50 ml of methanol, heated and dissolved, then 20 ml of 6M sodium hydroxide solution was added, and the mixture was refluxed for 1 hour.
[0072] The reaction solution was acidified to pH=2 with dilute hydrochloric acid. Filter, wash, and dry to obtain 20 g of white solid powder, the total yield of the two steps is 93%. 1 H-NMR (CDCl 3 )...
Embodiment 2
[0082] Preparation of N-[2-butyl-3-[4-[3-chloropropoxy]phenyl]-5-benzofuryl]-methanesulfonamide
[0083] 3 g of N-(2-butyl-5-benzofuryl)methanesulfonamide was dissolved in 15 ml of dichloromethane, and 3 g of aluminum trichloride was added in batches. At room temperature, 2.33 g of 4-(3-chloropropoxy)benzoyl chloride prepared as described in Example 1 was added, and the reaction was stirred for 1 hour. After the reaction is complete, pour it into 150ml of ice water, stir, separate the liquids, and after drying, concentrate to dryness under reduced pressure to obtain N-[2-butyl-3-[4-[3-chloropropoxy]phenyl]- 4.1 g of 5-benzofuryl]-methanesulfonamide, the yield was 88.3%. The NMR was consistent with Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com