Chromium carbide modified iron-based metal bipolar plate and preparation method thereof
A metal bipolar plate and chromium carbide technology, which is applied in metal material coating technology, battery electrodes, electrochemical generators, etc., can solve the problems of high cost of noble metal coating, reduced service life of polymer electrolyte membrane fuel cells, and discomfort Focusing on low-cost production and other issues, to achieve low material and processing costs, realize mass industrial production and large-scale market applications, and achieve low cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
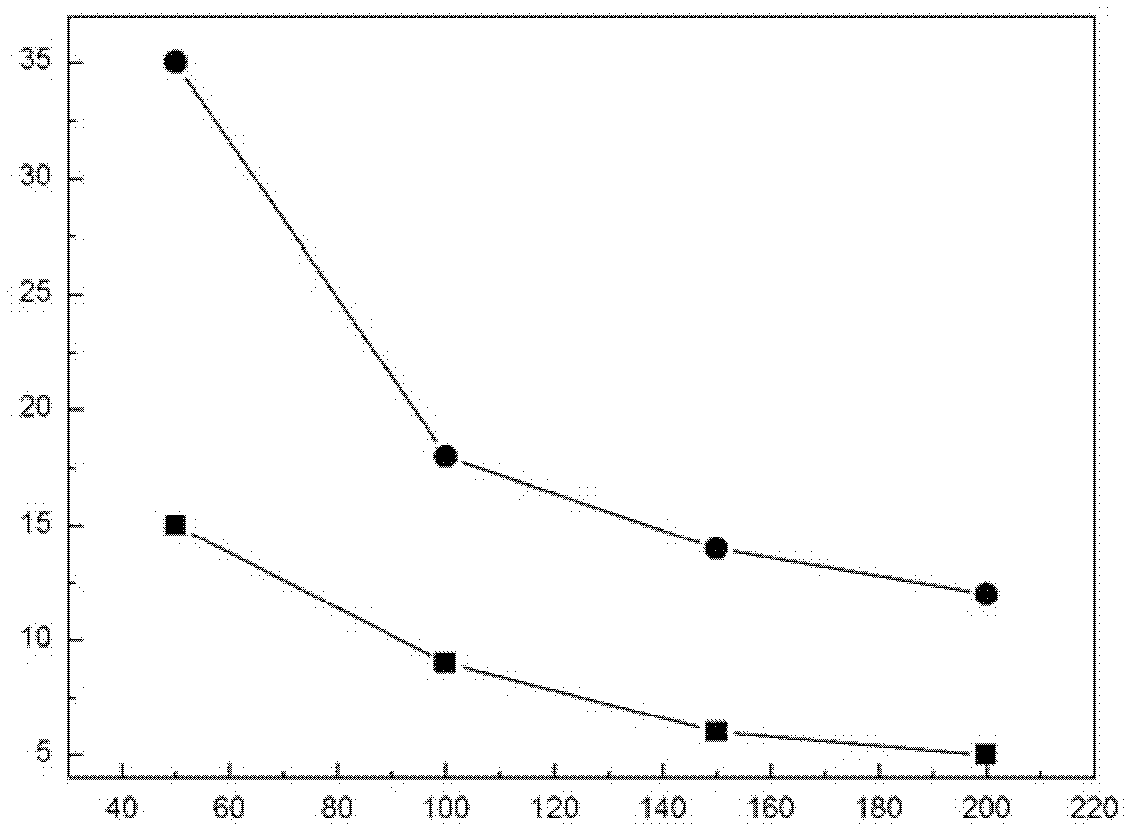
[0018] Plasma carburizing and thermal reaction deposition and diffusion composite surface modification technology are used to treat iron-based alloys (0.42-0.50% C, 0.17-0.37% Si, 0.35-0.80% Mn, Cr≤0.25%, Ni≤0.30%, Cu≤ 0.25%, P2 , the vacuum degree is 100Pa, the carburizing time is 2h, and the reaction gas is methane. The temperature of thermal reaction deposition and diffusion is 500℃, and the diffusion time is 0.5h, the generated Cr 7 C 3 The layer thickness is 1 μm. The interfacial contact resistance of the iron-based alloy metal bipolar plate after plasma carburizing and thermal reaction deposition and diffusion compound surface modification is significantly reduced, slightly higher than that of graphite, such as figure 1 shown. The current density is less than 10μA / cm under the working conditions of simulated polymer electrolyte membrane fuel cells 2 .
Embodiment 2
[0020] Plasma carburizing and thermal reaction deposition and diffusion compound surface modification technology are used to treat iron-based alloys (0.17~0.23%C, 0.17~0.37%Si, 0.35~0.80%Mn, Cr≤0.25%, Ni≤0.30%, Cu≤ 0.25%, P2 , the vacuum degree is 880Pa, the carburizing time is 5h, and the reaction gas is acetylene. The temperature of thermal reaction deposition and diffusion is 750℃, the diffusion time is 20h, the generated Cr 3 C 2 +Cr 7 C 3 The layer thickness was 20 μm. The interfacial contact resistance of the iron-based alloy metal bipolar plate after plasma carburizing and thermal reaction deposition and diffusion compound surface modification is significantly reduced, slightly higher than that of graphite, such as figure 1 shown. The current density is less than 10μA / cm under the working conditions of simulated polymer electrolyte membrane fuel cells 2 .
Embodiment 3
[0022] Plasma carburizing and thermal reaction deposition and diffusion composite surface modification technology are used to treat iron-based alloys (0.07-0.14% C, 0.17-0.37% Si, 0.35-0.80% Mn, Cr≤0.25%, Ni≤0.30%, Cu≤ 0.25%, P2 , the vacuum degree is 1330Pa, the carburizing time is 8h, and the reaction gas is propane. The temperature of thermal reaction deposition and diffusion is 1000℃, and the diffusion time is 45h. The generated Cr 3 C 2 +Cr 23 C 6 The layer thickness was 30 μm. The interfacial contact resistance of the iron-based alloy metal bipolar plate after plasma carburizing and thermal reaction deposition and diffusion compound surface modification is significantly reduced, slightly higher than that of graphite, such as figure 1 shown. The current density is less than 10μA / cm under the working conditions of simulated polymer electrolyte membrane fuel cells 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com