Medicinal composition for eradicating helicobacter pylori and preparation method thereof
A technology of Helicobacter pylori and its composition is applied in the field of medicines and manufacturing for inhibiting Helicobacter pylori, can solve the problems of reducing the effectiveness of antibiotic eradication, poor drug absorption, and not as expected, and achieves the effect of improving the bioavailability rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Manufacturing method of suspension dosage form pharmaceutical composition for eradicating H.pylori
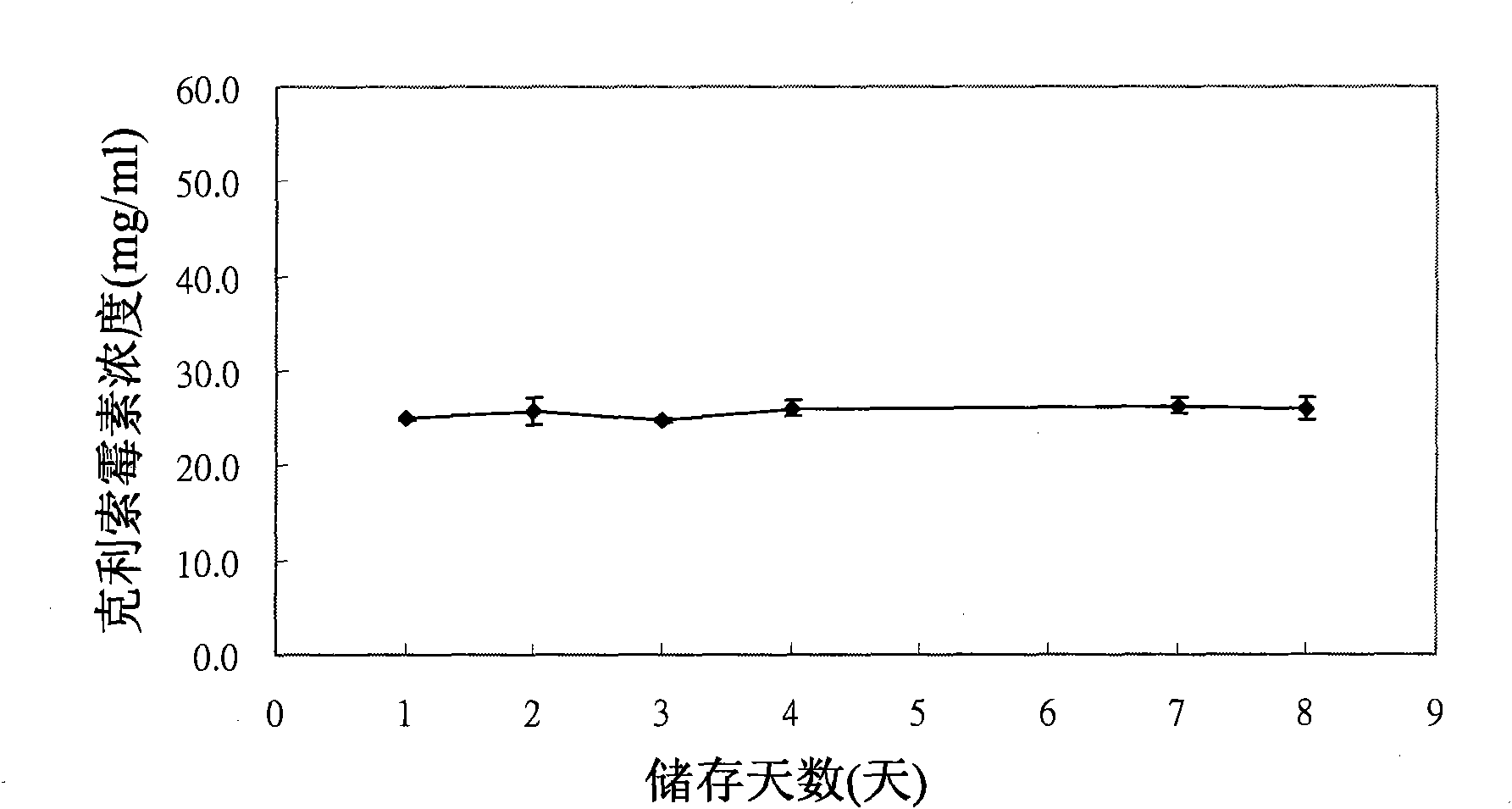
[0034] The active ingredients of the suspension formulation pharmaceutical composition for eradicating H. pylori in the embodiment of the present invention include 20 mg of famotidine, 500 mg of clarithromycin and 1 g of amoxicillin; 20mg of famotidine can be replaced by 290mg of ranitidine / bismuth citrate, where both famotidine and ranitidine are hydrogen Proton blocker (H 2 Blocker). The production method of the suspension dosage pharmaceutical composition for eradicating H. pylori in the embodiment of the present invention is exemplified by the production method of the pharmaceutical composition containing famotidine (famotidine), the pharmaceutical composition contains 20mg of famotidine (famotidine) , 500 mg of clarithromycin and 1 g of amoxicillin, Ac-Di-Sol as a disintegrating agent, colloidal silicon dioxide as a sliding agent, xanthan gum, carboxymethylcellu...
Embodiment 2
[0116] Manufacturing method of effervescent lozenge type pharmaceutical composition for eradicating H.pylori
[0117] The active ingredients of the effervescent lozenge-type medical composition for eradicating H. pylori in the embodiment of the present invention include 20 mg of omeprazole, 500 mg of clarithromycin and 1 g of amoxicillin, Sodium bicarbonate (sodium bicarbonate) and anhydrous citric acid (anhydrous citric acid) as a foaming agent, sodium benzoate (sodium propionate) as an antibacterial agent, and colloidal silicon dioxide (colloidal silicon dioxide) as a slip agent, as Suspending agent carboxymethylcellulose sodium (Carboxymethylcellulose Sodium) and hydroxypropylmethylcellulose (hydroxypropylmethylcellulose), and flavoring agent, its ingredient list and production process are as follows:
[0118]
Ingredients of Foaming Tablet Type Pharmaceutical Composition
theoretical weight
(mg) / 20ml
%
scope
Amoxycilli...
Embodiment 3
[0128] The manufacture method of the powder dosage form pharmaceutical composition for eradicating H.pylori:
[0129]
Components of powder dosage form pharmaceutical composition
theoretical weight
(mg) / 20ml
%
scope
Amoxycillin
101.6
11.5%
250~1000mg
Clithomycin
51.2
5.8%
125~500mg
4.0
0.5%
5~20mg
350.0
39.6%
15~75%
Carboxypropylmethylcellulose (HPMC)
42.0
4.7%
2~15%
Sanxianjiao
3.0
0.3%
0.2~15%
14.0
1.6%
0.02~1.5%
75.0
8.5%
1~25%
168.0
19.0%
5~25%
2.0
0.2%
0.1~8%
3.5
0.4%
0.2~5%
[0130] lemon powder
70.0
7.9%
2~15%
Total
884.3
100.0%
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com