Flucloxacilin sodium liposome injection
A technology of flucloxacillin sodium lipid and flucloxacillin sodium, which is applied in the field of pharmaceutical preparations, can solve the problems of failing to meet the quality requirements of the validity period, low liposome encapsulation efficiency, and unstable quality, and achieve low cost and reduced toxicity. Side effects, effects of improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 flucloxacillin sodium lipid microsphere injection
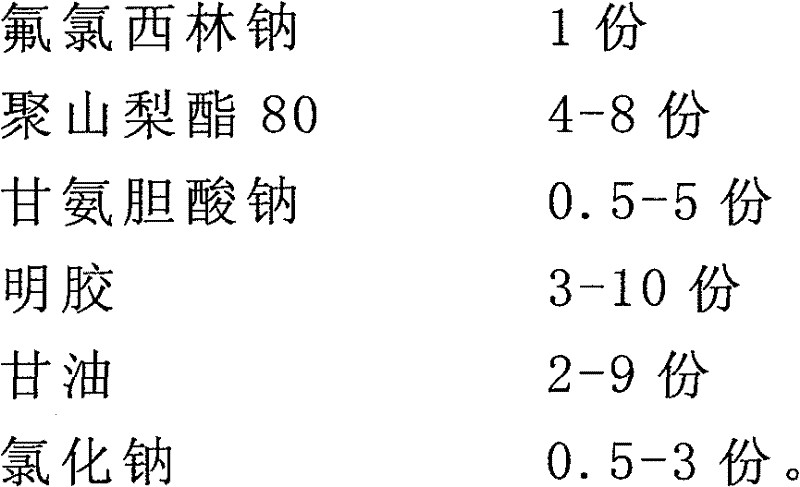
[0037] Prescription: (100 bottles)
[0038]
[0039]
[0040] Preparation Process:
[0041] (1) Dissolve 50g of flucloxacillin sodium, 100g of glycerin and 25g of sodium chloride in 2000ml of water for injection to obtain an aqueous phase;
[0042] (2) 200g polysorbate 80, 150g gelatin and 25g sodium glycocholate were dissolved in 2000ml volume ratio of n-hexane and isopropanol in a mixed solvent of 1:1 to obtain an oil phase;
[0043] (3) Slowly drop the water phase obtained above into the oil phase under stirring conditions, control the dropping speed to 3ml / min, stir for 10min after dropping, then transfer to a high-speed homogenizer and stir at a high speed for 3 times at a speed of 12000r / min, each 5min, to obtain a uniform white emulsion;
[0044] (4) Put the white emulsion into the spray dryer, adjust the spray conditions: the inlet temperature is 90°C, the outlet temper...
Embodiment 2
[0046] The preparation of embodiment 2 flucloxacillin sodium lipid microsphere injection
[0047] Prescription: (100 bottles)
[0048]
[0049] Preparation Process:
[0050] (1) 50g flucloxacillin sodium, 450g glycerol and 150g sodium chloride were dissolved in 4000ml water for injection to obtain the aqueous phase;
[0051] (2) 400g polysorbate 80, 500g gelatin and 250g sodium glycocholate are dissolved in 4000ml of mixed solvent of n-hexane and Virahol with a volume ratio of 1:1 to obtain an oil phase;
[0052] (3) Slowly drop the water phase obtained above into the oil phase under stirring conditions, control the dropping speed to 8ml / min, stir for 30min after dropping, then transfer to a high-speed homogenizer and stir at a high speed for 5 times at a speed of 12000r / min, each 10min, to obtain a uniform white emulsion;
[0053] (4) Put the white emulsion into the spray dryer, adjust the spray conditions: the inlet temperature is 80°C, the outlet temperature is about...
Embodiment 3
[0055] The preparation of embodiment 3 flucloxacillin sodium lipid microsphere injection
[0056] Prescription: (100 bottles)
[0057]
[0058] Preparation Process:
[0059] (1) 100g flucloxacillin sodium, 200g glycerol and 50g sodium chloride are dissolved in 3000ml water for injection to obtain the aqueous phase;
[0060] (2) 400g polysorbate 80, 300g gelatin and 50g sodium glycocholate were dissolved in 3000ml volume ratio of n-hexane and isopropanol in a mixed solvent of 1:1 to obtain an oil phase;
[0061] (3) Slowly drop the water phase obtained above into the oil phase under stirring conditions, control the dropping speed to 5ml / min, stir for 20min after dropping, then transfer to a high-speed homogenizer and stir at a high speed for 4 times at a speed of 12000r / min, 8min each time, to obtain a uniform white emulsion;
[0062] (4) Put the white emulsion into the spray dryer, adjust the spray conditions: the inlet temperature is 85°C, the outlet temperature is abo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com