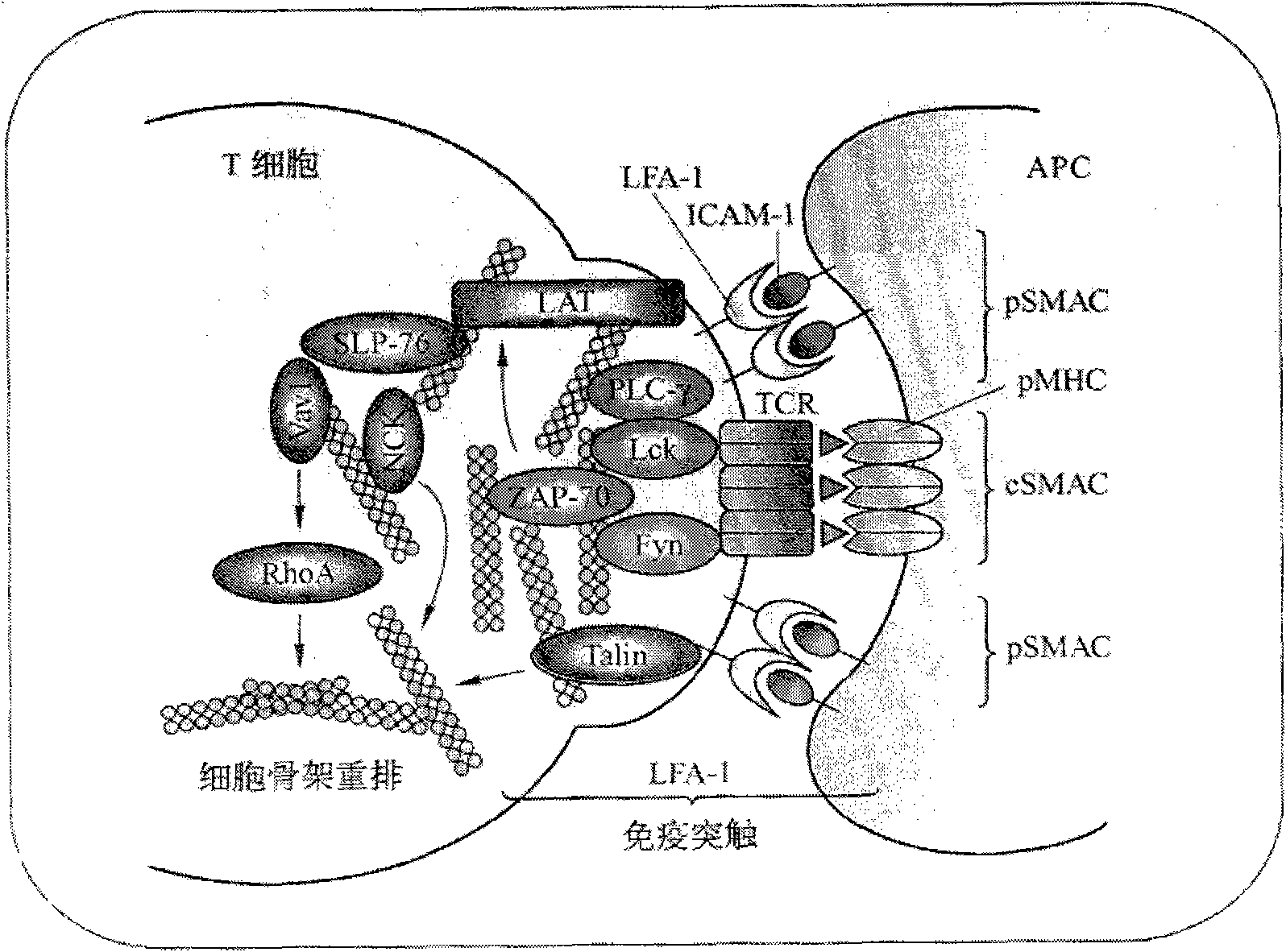
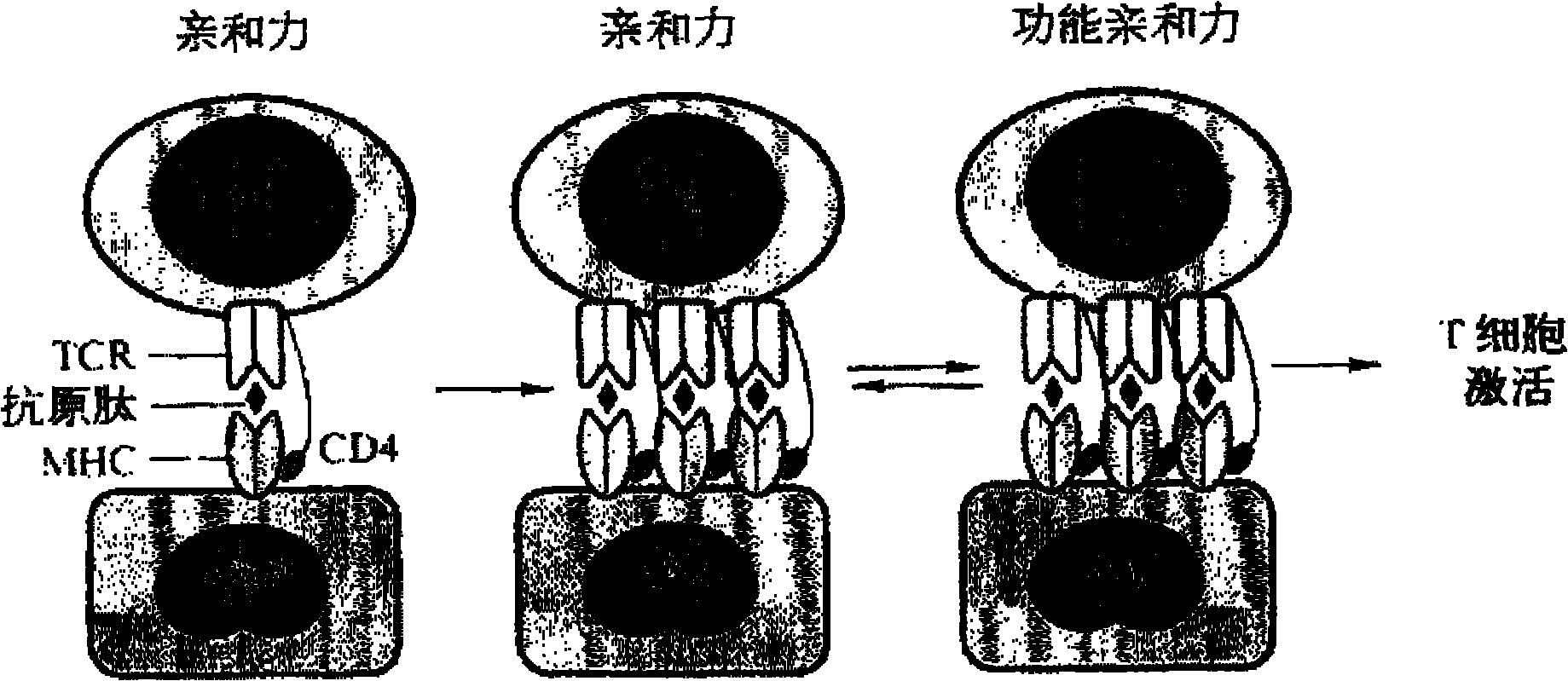
T-cell immune balance peptide
A cellular immunity and balance technology, applied in the direction of peptides, hybrid peptides, specific peptides, etc., can solve the problems of chronic infection that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Preparation of T cell immune balance peptides from animal tissues
[0109] 1. Select healthy suckling pigs or non-lactating newborn cows, take fresh livers, hearts, kidneys and spleens, etc., wash them with water for injection, and shred or chop them at about 24°C;
[0110] 2. Add sterilized water for injection according to weight: volume ratio of 1:1, and homogenate repeatedly for 10 minutes at low temperature (4°C) to obtain a homogenate; put the homogenate into a low temperature of minus 60 to 70 degrees to freeze After freezing, quickly heat up the quick-thaw solution to 85°C and keep the temperature constant for 10 minutes to achieve thermal denaturation of macromolecular proteins and remove macromolecular proteins;
[0111] 3. After heat denaturation, centrifuge at 4°C, 3000 rpm for 20 minutes, discard the precipitate, and take the supernatant;
[0112] 4. Graded ultrafiltration and virus inactivation: the centrifugal supernatant is subjected to graded ultrafiltr...
Embodiment 2
[0115] Preparation of T cell immune balance peptide by chemical synthesis
[0116] The preparation of polypeptides uses the Fmoc solid-phase peptide synthesis method. First, an amino acid protected by an Fmoc group on the α-amino group is connected to an insoluble carrier through an arm, and then the α-amino group is deprotected, and the amino acid is washed with a solution- Arm - Resin. The second pre-activated α-amino protected amino acid is connected through a coupling reaction. After the condensation reaction is completed, it is washed with a solution, and the deprotection and coupling are repeated until the target peptide is obtained. Finally the peptide-arm-resin is cleaved. Peptide purification, using HPLC method, the crude peptide product is separated and purified by C18 high-pressure column, and the required effluent is tracked and collected by liquid chromatography, the sample peaks are combined, desalted, and freeze-dried to obtain a high-quality peptide.
[0117]...
Embodiment 3
[0154] ELISPOT method to detect the inhibitory effect of synthetic peptide mixture and PHGF on HBcAg-induced IFN-gamma
[0155] Using HBcAg to stimulate human peripheral blood mononuclear cells (PBMC) to induce IFN-gamma, observe the effect of different concentrations of synthetic peptide mixture and PHGF on the production of IFN-gamma, so as to reflect their regulatory effect on T cell specific immunity
[0156] experimental reagent
[0157] RPMI1640 medium and fetal bovine serum, lymphocyte separation fluid, anti-human CD3, HBcAg, synthetic decapeptide (mentioned above), PHGF, ELISPOT kit, solid heparin sodium anticoagulant, double distilled water, sterile ionized water.
[0158] experimental method
[0159] 6 healthy subjects were required to fast for at least 6 hours before blood collection. Take 5ml of peripheral blood from volunteers or patients under aseptic conditions with high-pressure sterilized blood collection tubes, anticoagulate with 100 units of heparin sodiu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com