Fotemustine solid lipid nanoparticle and preparation method thereof
A technology of solid lipid nanometer and formustine, which is applied in the field of pharmacy, can solve the problems of difficult industrial production of instruments and equipment, poor product stability, and limited continuous application, so as to improve the chemical stability and curative effect of the drug, with low cost, The effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
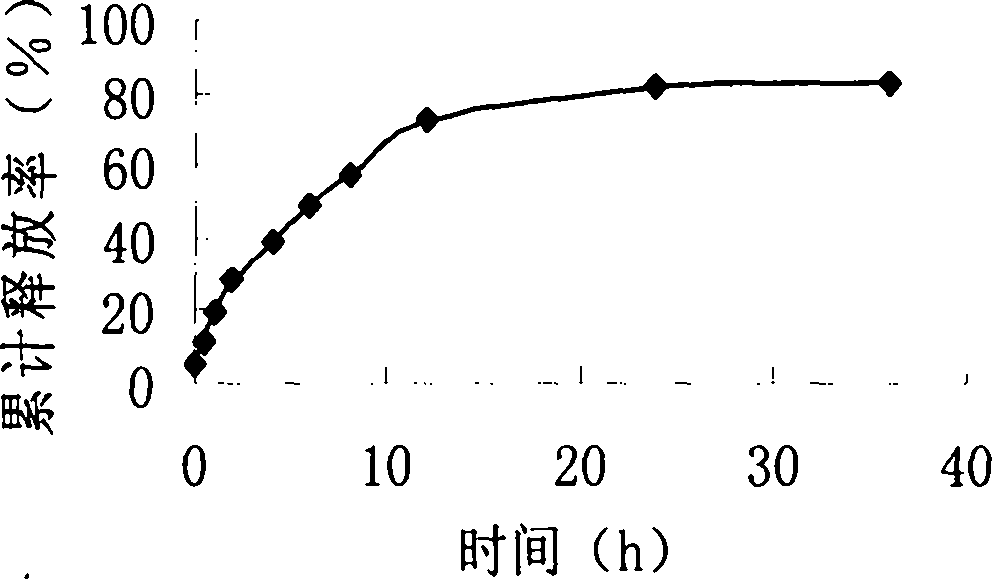
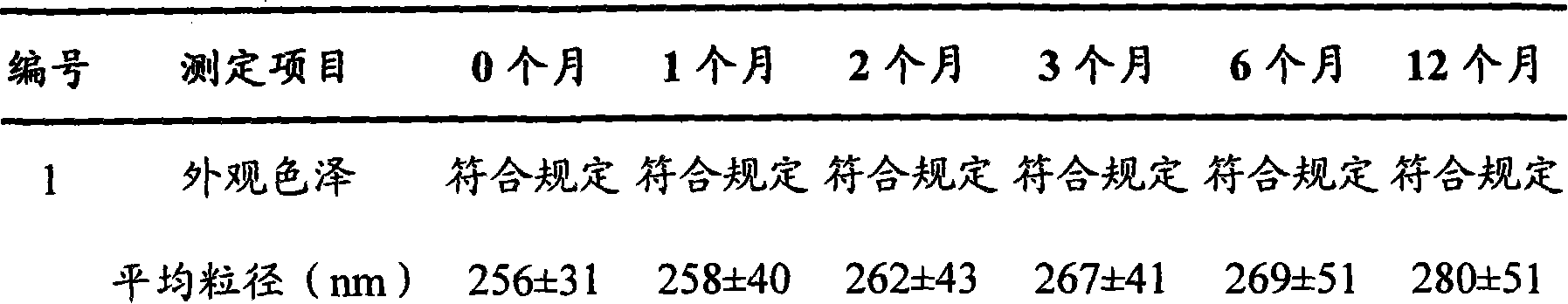
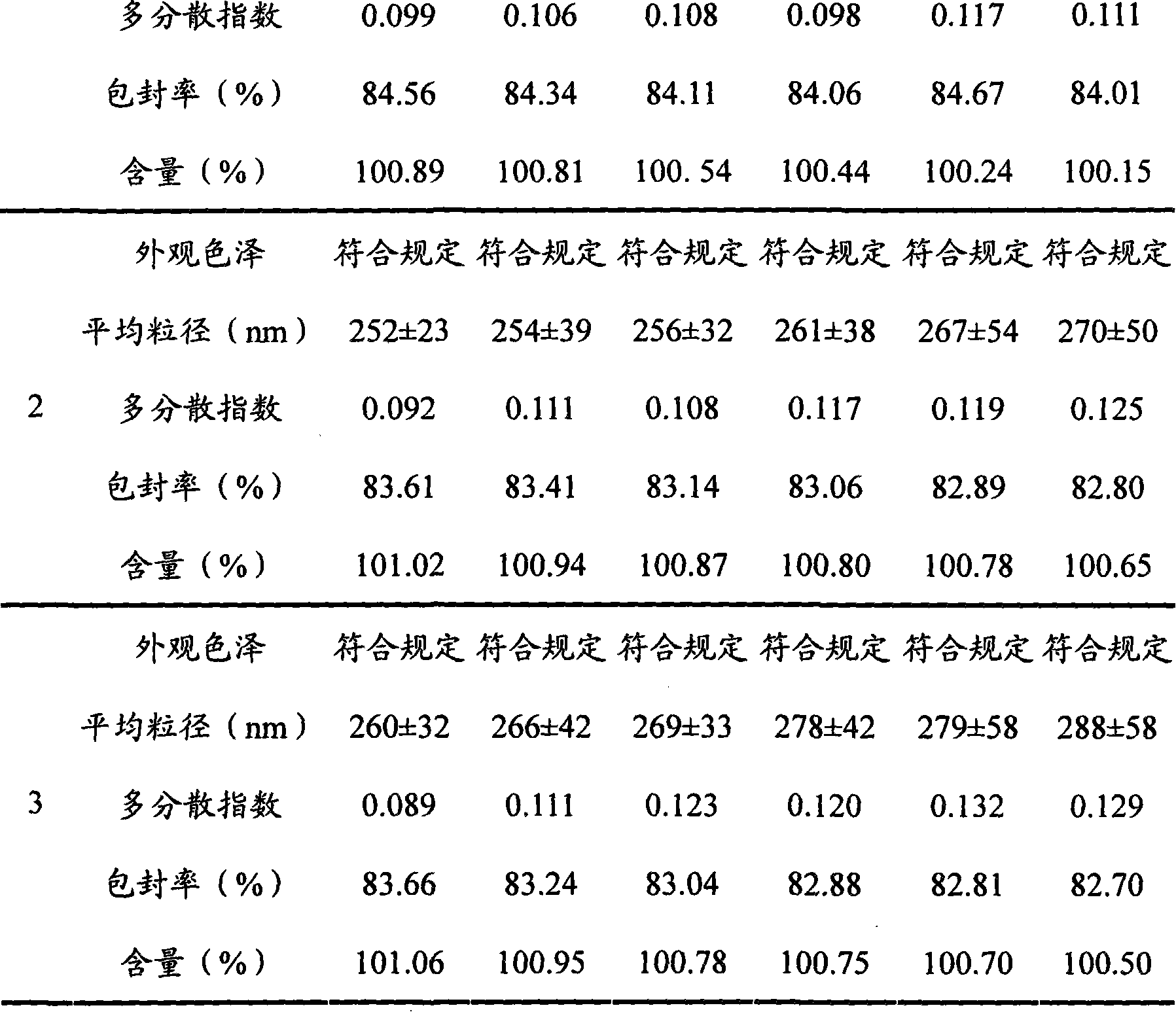
[0033] Take by weighing 208mg formustine, 1200mg stearic acid and 1000mg lecithin and dissolve in 20ml of methylene chloride, evaporate to dryness under reduced pressure at 40°C to form a lipid film, add 4ml of Tween-80 (2.5%) , ultrasonically dispersed for 30 minutes (ultrasonic power 600w), and the solid lipid nanoparticle suspension was obtained, which was stored at 2°C for later use. Its average particle size is 210nm, all particles are below 500nm, and the particle size distribution is narrow, showing that the size of solid lipid nanoparticles is relatively uniform; the solid lipid nanoparticle suspension can be stable for several days at room temperature and can Stable for 12 months, no precipitation was observed during storage.
Embodiment 2
[0035] Weigh 624mg of formustine, 6240mg of glyceryl monostearate and 3000mg of soybean lecithin into a 50ml conical flask with a stopper, add 10ml of ethanol: acetone (1:1) mixed solvent, ultrasonically dissolve it fully and heat to 60 °C, forming the organic phase. Another 500mg of Poloxamer-188 was dissolved in 30ml of double distilled water to form the water phase. Use a syringe to slowly inject the organic phase into the 60°C aqueous phase stirred at 1000r / min, and continue stirring until a translucent system is formed. The organic solvent was evaporated under reduced pressure at 40° C., the system was concentrated to 12 ml, and cooled to room temperature to obtain a solid lipid nanoparticle suspension.
Embodiment 3
[0037] Dissolve 0.624g of formustine, 12.48g of tripalmitin, 1.2g of stearyl alcohol, and 6.24g of dipalmitoylphosphatidylcholine in 20ml of ethanol, ultrasonically dissolve and heat to (50±2)°C to form an organic phase . Another 1g of myrj 53 was added into 40ml of double-distilled water, and ultrasonically dissolved to form an aqueous phase. Slowly inject the organic phase into the constant temperature water phase of (50±2)°C stirred at 1000r / min to form colostrum, and continue to stir for about 2-3 hours to completely evaporate the organic solvent and concentrate the system to about 2ml. Quickly mix the obtained translucent emulsion into another 10ml ice-water phase stirred at 1000r / min at 0-2°C, continue to stir for 2 hours, and then homogenize 5 times in a high-pressure homogenizer (homogeneous pressure is 15000psi) , to obtain solid lipid nanoparticle suspension.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com