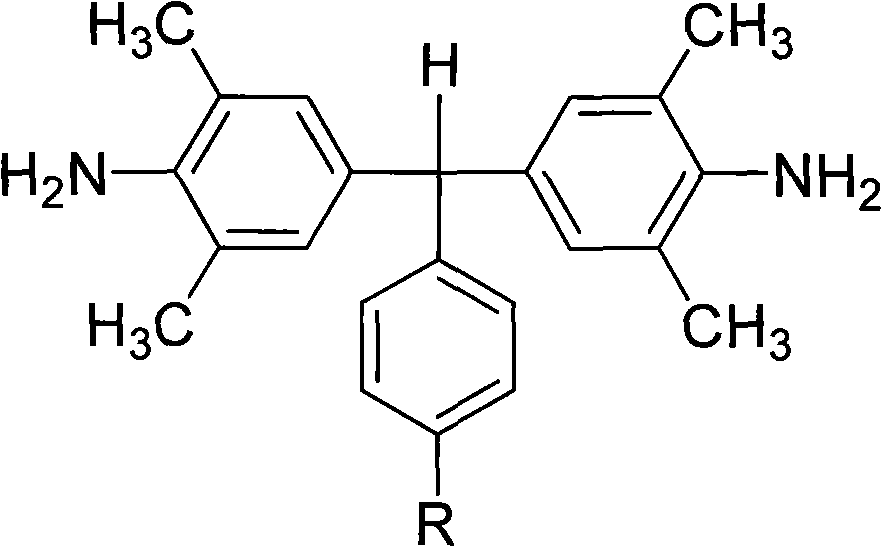
Polyalkyl-substituted aromatic diamine monomer and preparation and application thereof
A technology of aromatic diamine and polyalkyl group, which is applied in the field of polyalkyl substituted aromatic diamine monomer and its preparation and application, can solve the problems of limited solubility, loss, and reduced high temperature resistance of polyimide, and achieve Excellent mechanical properties, low cost, and improved solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
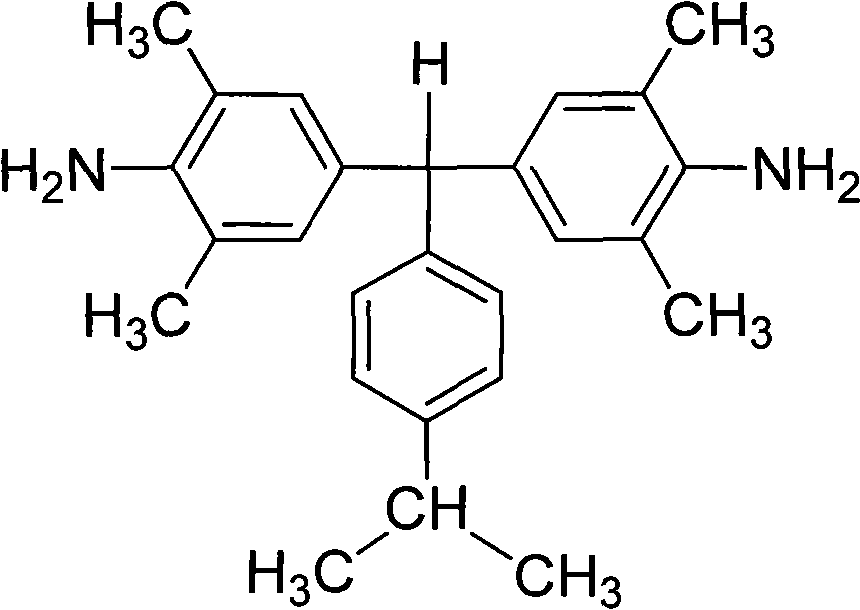
[0035] Preparation of α,α-(4-amino-3,5,-dimethylphenyl)-4'-isopropyltoluene:
[0036] Under the protection of nitrogen, 100 parts of 2,6-dimethylaniline was heated to 110°C under the protection of nitrogen, and then a mixed solution of 35 parts of 4-isopropylbenzaldehyde and 20 parts of hydrochloric acid was added dropwise thereto. After the dropwise addition, the reactant mixture was heated to reflux at 155°C for 12 hours. Cool to 60°C, add dropwise to neutral with 10% aqueous sodium hydroxide solution. Excessive ethanol was added to produce a large amount of precipitate; filtered, washed with methanol, dried, and further separated and purified by silica gel column chromatography to obtain a white diamine product with a yield of about 60-80% and a melting point of 157-158°C. FT-IR(KBr): 3440, 3368, 3226, 2961, 2925, 2866, 1623, 1600, 1488, 1303, 1152, 1022, 883, 843 and 653cm -1 . 1 H-NMR (400MHz, DMSO-d 6 , ppm): 7.09 (d, J = 8.0Hz, 2H), 6.96 (d, J = 8.0Hz, 2H), 6.52 (s,...
Embodiment 2
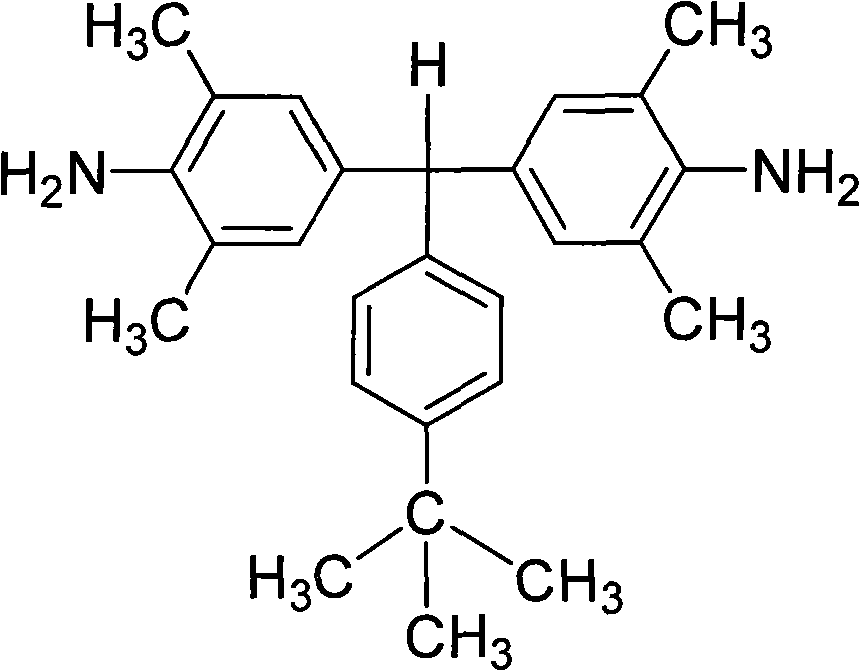
[0038] α, α-(4-amino-3,5,-dimethylphenyl)-4'-tert-butyltoluene preparation:
[0039] Under the protection of nitrogen, 100 parts of 2,6-dimethylaniline was heated to 110°C under the protection of nitrogen, and then a mixed solution of 30 parts of 4-tert-butylbenzaldehyde and 5 parts of hydrochloric acid was added dropwise thereto. After the dropwise addition, the reactant mixture was heated to reflux at 160°C for 15 hours. Cool to 60°C, add dropwise to neutral with 10% aqueous sodium hydroxide solution. Excessive ethanol was added to produce a large amount of precipitate; filtered, washed with methanol, dried, and further separated and purified by silica gel column chromatography to obtain a white diamine product with a yield of about 60-80% and a melting point of 184-185°C. FT-IR(KBr): 3446, 3366, 2966, 2904, 2865, 1623, 1489, 1306, 1150, 1022, 841, 843 and 651cm -1 . 1 H-NMR (400MHz, DMSO-d 6 , ppm): 7.24(d, J=8.2Hz, 2H), 6.98(d, J=8.2Hz, 2H), 6.54(s, 4H), 5.03(s, 1H), 4...
Embodiment 3
[0041] Add 1.5mmol of polyalkylated aromatic diamine and 1.5mmol of diphenyl ether tetraacid dianhydride to a 50ml three-neck round-bottomed flask that is dry and ventilated with nitrogen, and then add 15ml of m-cresol solvent (the solid content of the system is between 5 and 20 %), then add about 0.4g of isoquinoline as a catalyst, raise the temperature of the reaction system to 100°C for 2-3 hours, then raise the temperature to 190-200°C for about 10 hours, and pour the polymer solution into 300ml after cooling to 120°C In methanol or ethanol, the precipitate was collected by filtration, washed twice with boiling water, and vacuum-dried at 150°C to obtain a white fibrous polyimide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com