Omeprazole sodium lyophilized preparation for injection and preparation method thereof
A technology of omeprazole sodium and freeze-dried preparations, applied in the field of medicine, can solve the problems of being difficult to be stabilized and the properties of omeprazole sodium are unstable, and achieve improved drug release rate, small particle size, and Zeta potential. improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
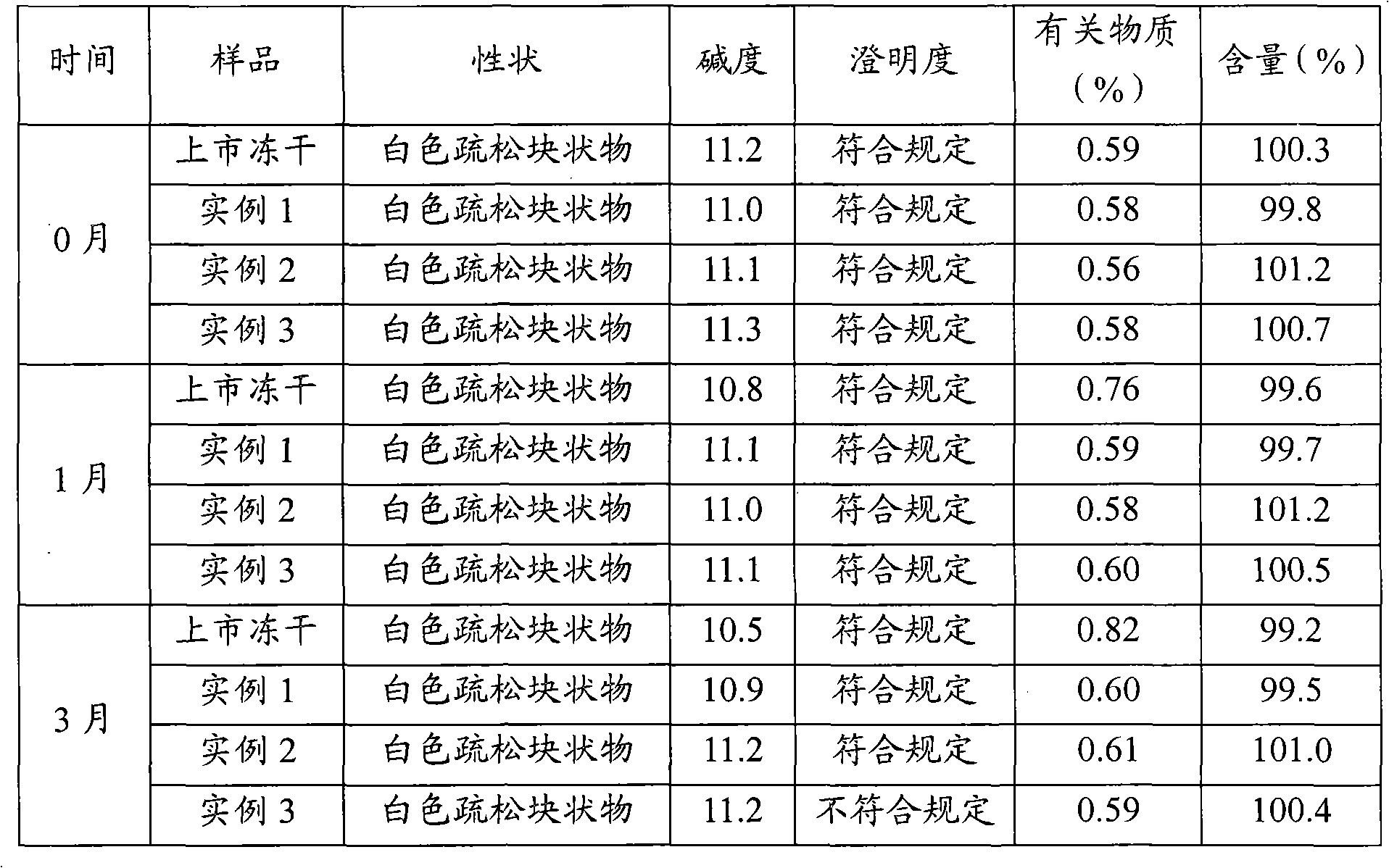
Examples
example 1
[0020] raw material:
[0021] Omeprazole Sodium 20g
[0022] Dextran 20g
[0023] Povidone K15 5g
[0025] Polymethylmethacrylate 40g
[0026] Lactose 10g.
[0027] Preparation Process
[0028] (1) Take by weighing 20g omeprazole sodium, 20g dextran, 5g povidone K15, add 1000ml of water for injection to dissolve, adjust the pH value to 2.5 with the hydrochloric acid solution of 1mol / L, stir;
[0029] (2) Slowly add 40g polymethyl methacrylate under electromagnetic stirring, continue to stir for 3 hours at room temperature, add 5g of sodium sulfite, continue to stir for 3 hours, adjust the pH value to 12 with 1mol / L sodium hydroxide solution, stir Uniform;
[0030] (3) Add 10 g of lactose, stir to dissolve, then add 0.1% of the total volume of the solution with charcoal for needles, stir at room temperature for 10 minutes, filter for decarburization, and then fine filter with a 0.22 μm microporous membrane to obtain omeprazole sodium nanopartic...
example 2
[0033] raw material:
[0034] Omeprazole Sodium 40g
[0035] Dextran 200g
[0036] Povidone K30 60g
[0037] Sodium sulfite 40g
[0038] Polymethylmethacrylate 400g
[0039] Glucose 500g.
[0040] Preparation Process
[0041] (1) Take by weighing 40g omeprazole sodium, 200g dextran and 60g povidone K30, add 3000ml of water for injection to dissolve, adjust the pH value to 2.8 with the hydrochloric acid solution of 1mol / L, stir;
[0042] (2) Slowly add 400g polymethyl methacrylate under electromagnetic stirring, continue stirring for 3 hours at room temperature, add 40g sodium sulfite, continue stirring for 3 hours, adjust the pH value to 9 with 1mol / L sodium hydroxide solution, stir Uniform;
[0043] (3) Add 500 g of glucose, stir to dissolve, then add 0.3% of the total volume of the solution with charcoal for needles, stir at room temperature for 30 minutes, filter for decarburization, and then fine filter with a 0.22 μm microporous membrane to obtain omeprazole sodium...
example 3
[0046] raw material:
[0047] Omeprazole Sodium 30g
[0048] Dextran 100g
[0049] Dextran 40 20g
[0050] Sodium sulfite 40g
[0051] Polymethylmethacrylate 200g
[0052] Mannitol 300g.
[0053] Preparation Process
[0054] (1) Take by weighing 30g omeprazole sodium, 100g dextran and 20g dextran 40, add 2000ml of water for injection to dissolve, adjust the pH value to 2.2 with the hydrochloric acid solution of 1mol / L, stir;
[0055] (2) Slowly add 200g polymethyl methacrylate under electromagnetic stirring, continue stirring for 3 hours at room temperature, add 30g sodium sulfite, continue stirring for 3 hours, adjust the pH value to 10 with 1mol / L sodium hydroxide solution, stir Uniform;
[0056] (3) Add 300 g of mannitol, stir to dissolve, then add 0.2% of the total volume of the solution with charcoal for needles, stir at room temperature for 20 minutes, filter for decarburization, and then fine filter with a 0.22 μm microporous membrane to obtain omeprazole sodium na...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com