Method for removing impurity element Na and S in precursor cobalt salts of cobaltosic oxide
A technology of cobalt tetroxide and impurity elements, applied in the direction of cobalt oxide/cobalt hydroxide, etc., can solve the problems of increased hydrolysis and dissolution of cobalt salts, increased treatment capacity of cobalt-containing wastewater, and inability to effectively remove them. Chemical properties, inhibition of hydrolysis and dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
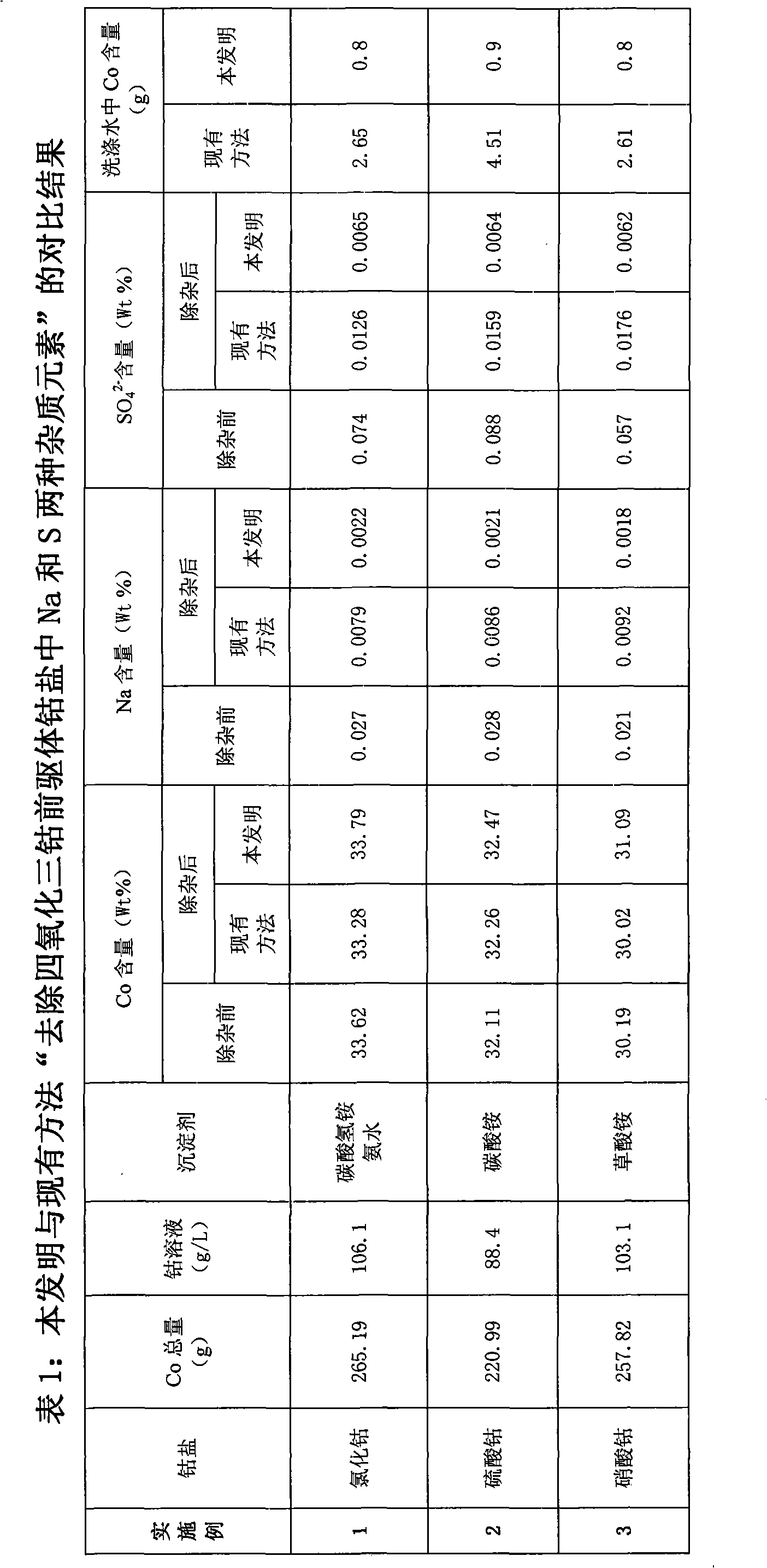
[0022] a) In a 5L glass container, take industrial-grade cobalt chloride and prepare 2500ml of cobalt chloride solution of 1.8mol / l with deionized water, stir, adjust the pH value to 0.5 with hydrochloric acid, heat to 30°C, add 10 % sodium hypochlorite aqueous solution 5ml, continue stirring for 45 minutes, slowly add 2% dilute ammonia water dropwise to adjust its pH value to 4.5, heat to 90°C, add 3g of industrial grade ammonium fluoride, and keep warm for 60 minutes. After the heat preservation is completed, 2 ml of 23% ammonium sulfide solution is added, and the heat preservation is carried out for 60 minutes. Stop heating and stirring, let it stand for 24 hours, and filter to obtain pure cobalt solution.
[0023] b) Heating the cobalt solution obtained in step a) to 75°C, adding 15% ammonium bicarbonate and 20% ammonia water mixed solution, the amount of ammonium bicarbonate added is 2 times of the total molar amount of cobalt in the solution, ammonia water The amount is...
Embodiment 2
[0030]a) In a 5L glass container, take industrial-grade cobalt sulfate and use deionized water to prepare 2500ml of cobalt sulfate solution of 1.5mol / l, stir, adjust its pH value to 1.5 with 5% dilute sulfuric acid, and heat to 50°C. Add 3ml of 10% industrial hydrogen peroxide, continue to stir for 60 minutes, then heat to 80°C, adjust the pH value to 4.8 with 2% sodium hydroxide, heat to 90°C, and keep warm for 0.5h. After the heat preservation is completed, 3.2 g of industrial grade ammonium fluoride is added, and the heat preservation is carried out for 30 minutes. After the heat preservation is completed, 2 ml of 25% sodium sulfide solution is added, and the heat preservation is carried out for 60 minutes. Stop heating and stirring, let it stand for 6h, and filter to obtain pure cobalt solution.
[0031] b) Heating the cobalt solution obtained in step a) to 90°C, adding 15% aqueous sodium carbonate solution, the amount of sodium carbonate added is 1.3 times the total mola...
Embodiment 3
[0038] a) In a 5L glass container, take industrial-grade cobalt nitrate and prepare 2500ml of cobalt nitrate solution of 1.75mol / l with deionized water, stir, adjust the pH value to 4.5 with hydrochloric acid, heat to 30°C, add 25% 3ml of industrial hydrogen peroxide, continue to stir for 45 minutes, then heat to 80°C, slowly add ammonia water dropwise to adjust the pH value to 4.8, heat to 90°C, and keep warm for 30 minutes. After the heat preservation is completed, 3.2 g of industrial grade ammonium bifluoride is added, and the heat preservation is carried out for 60 minutes. After the heat preservation is completed, 2 ml of 23% ammonium sulfide solution is added, and the heat preservation is carried out for 60 minutes. Stop heating and stirring, let it stand for 12 hours, and filter to obtain pure cobalt solution.
[0039] b) Heating the cobalt solution obtained in step a) to 75°C, adding 15% ammonium oxalate aqueous solution, the addition of ammonium oxalate is 1.2 times ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com